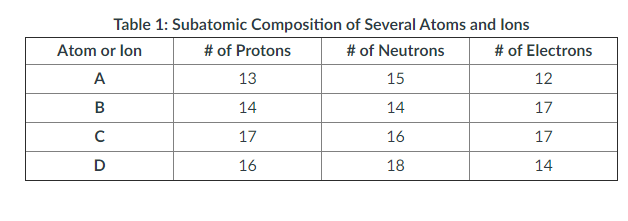
Describe the structure of an atom. What are atoms composed of and how are the individual parts of an atom arranged? Include the specific properties of each atomic subparticle and an illustration as part of your explanation. In the table above, which atom or ion is the heaviest? Support your claim using what you know about the different subatomic particles. In the table above, which atom or ion has the most negative charge? Support your claim using what you know about the different subatomic particles.
Describe the structure of an atom. What are atoms composed of and how are the individual parts of an atom arranged? Include the specific properties of each atomic subparticle and an illustration as part of your explanation.
In the table above, which atom or ion is the heaviest? Support your claim using what you know about the different subatomic particles.
In the table above, which atom or ion has the most negative charge? Support your claim using what you know about the different subatomic particles.
Describe what would happen to Atomic/Ion A if it lost an electron. What would happen if it lost a proton? A neutron? How would it change? Include reasons supporting your prediction for each.
Construct an atomic particle with the same charge as atom A but a different mass.
Construct an atomic particle with the same mass as atom A but a different charge.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









