description that an atom subjected to a high voltage 1.1. suggests the becomes charged because it's made of some positive material with small bits of negative material embedded in it, like raisins in a pudding. The high voltage rips the negative bits out, leaving the positive material behind, and making the once neutral atom charged. After studying how many different elements absorb X-rays, Harry finds that the atomic number of the element is always proportional to the square root of the wavelength of X-rays absorbed by the element, an that in the late 19th and early 20th example of the kind of centuries hinted at the structure of the atom. Joseph has found that when he passes a high voltage current through an evacuated tube, he can make a phosphor coating on one end of the tube that if a metal shape is put in the One day he makes the coating 0 0 0
description that an atom subjected to a high voltage 1.1. suggests the becomes charged because it's made of some positive material with small bits of negative material embedded in it, like raisins in a pudding. The high voltage rips the negative bits out, leaving the positive material behind, and making the once neutral atom charged. After studying how many different elements absorb X-rays, Harry finds that the atomic number of the element is always proportional to the square root of the wavelength of X-rays absorbed by the element, an that in the late 19th and early 20th example of the kind of centuries hinted at the structure of the atom. Joseph has found that when he passes a high voltage current through an evacuated tube, he can make a phosphor coating on one end of the tube that if a metal shape is put in the One day he makes the coating 0 0 0
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 10PS: In 1886 Eugene Goldstein observed positively charged particles moving in the opposite direction to...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Solve correctly please.
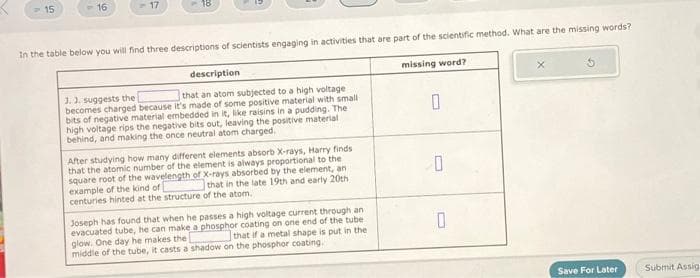
Transcribed Image Text:-15
16
-17
18
In the table below you will find three descriptions of scientists engaging in activities that are part of the scientific method. What are the missing words?
missing word?
description
3.3. suggests the
that an atom subjected to a high voltage
becomes charged because it's made of some positive material with small
bits of negative material embedded in it, like raisins in a pudding. The
high voltage rips the negative bits out, leaving the positive material
behind, and making the once neutral atom charged.
After studying how many different elements absorb X-rays, Harry finds
that the atomic number of the element is always proportional to the 1
square root of the wavelength of X-rays absorbed by the element, an
that in the late 19th and early 20th
example of the kind of
centuries hinted at the structure of the atom.
Joseph has found that when he passes a high voltage current through an
evacuated tube, he can make a phosphor coating on one end of the tube
that if a metal shape is put in the
glow. One day he makes the
middle of the tube, it casts a shadow on the phosphor coating.
0
0
0
Save For Later
Submit Assig
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning