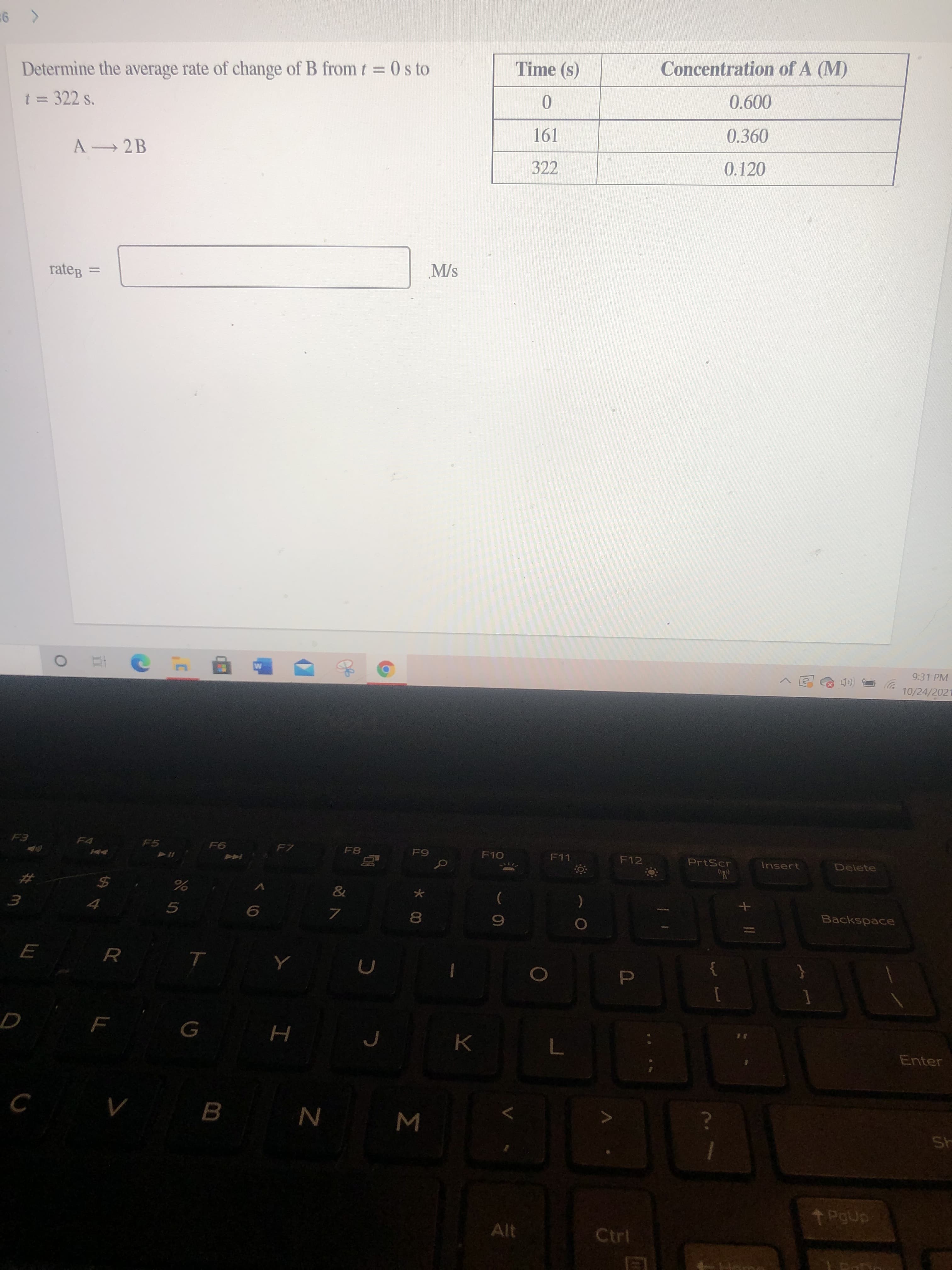
Determine the average rate of change of B from t = 0 s to Time (s) Concentration of A (M) %3D t = 322 s. 0.600 %3D 161 0.360 A 2B 322 0.120 rateg = M/s
Determine the average rate of change of B from t = 0 s to Time (s) Concentration of A (M) %3D t = 322 s. 0.600 %3D 161 0.360 A 2B 322 0.120 rateg = M/s
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 36E: From the given data, use a graphical method to determine the order and rate constant of the...
Related questions
Question
Transcribed Image Text:工
Time (s)
Concentration of A (M)
Determine the average rate of change of B from t = 0 s to
t 3D 322 s.
161
0.360
A 2 B
322
0.120
M/s
rateg
%3D
9:31 PM
10/24/2021
F4
F5
F7
F8
F10
F11
F12
PrtScr
Insert
Delete
%23
%24
%
Backspace
R.
1
Enter
N
tPgUp
Alt
Ctrl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning