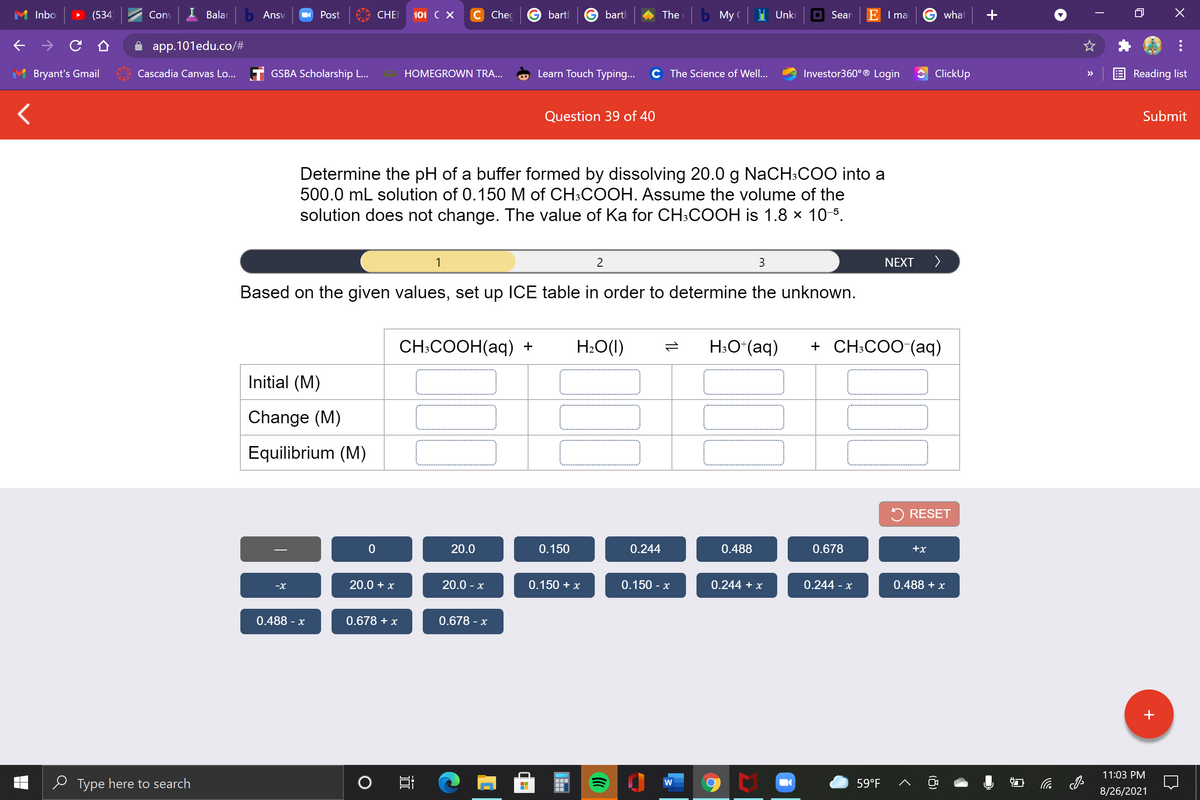
Determine the pH of a buffer formed by dissolving 20.0 g NaCH:COO into a 500.0 mL solution of 0.150 M of CH:COOH. Assume the volume of the solution does not change. The value of Ka for CH:COOH is 1.8 × 10-5. 3 NEXT > Based on the given values, set up 1CE table in order to determine the unknown. CH:COOH(aq) + H:O(1) H:O*(aq) + CH:COO (aq) Initial (M) Change (M) Equilibrium (M)
Determine the pH of a buffer formed by dissolving 20.0 g NaCH:COO into a 500.0 mL solution of 0.150 M of CH:COOH. Assume the volume of the solution does not change. The value of Ka for CH:COOH is 1.8 × 10-5. 3 NEXT > Based on the given values, set up 1CE table in order to determine the unknown. CH:COOH(aq) + H:O(1) H:O*(aq) + CH:COO (aq) Initial (M) Change (M) Equilibrium (M)
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 49A
Related questions
Question
Transcribed Image Text:Inbo
(534)
Conv
I Balar
b Ansv
Post
CHE
101 C X
с Chec
bartl
bartl
The
b My C
Unkr
O Sear
E I ma
G what
app.101edu.co/#
Bryant's Gmail
Cascadia Canvas Lo... T GSBA Scholarship L...
HOMEGROWN TRA...
Learn Touch Typing...
C The Science of Well...
Investor360° ® Login
ClickUp
Reading list
>>
Question 39 of 40
Submit
Determine the pH of a buffer formed by dissolving 20.0 g NaCH:COO into a
500.0 mL solution of 0.150 M of CH:COOH. Assume the volume of the
solution does not change. The value of Ka for CH:COOH is 1.8 x 10-5.
1
2
NEXT >
Based on the given values, set up ICE table in order to determine the unknown.
CH:COOH(аq) +
H2O(1)
H;O*(aq)
+ CH:COO-(aq)
Initial (M)
Change (M)
Equilibrium (M)
5 RESET
20.0
0.150
0.244
0.488
0.678
+x
20.0 + x
20.0 - x
0.150 + x
0.150 - x
0.244 + x
0.244 -
0.488 + x
-X
0.488 - x
0.678 + x
0.678 - x
+
11:03 PM
e Type here to search
59°F
W
8/26/2021
(8)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
