Determine the Rate and Rate Constant CK). Equation 1: 2I'cae) + Sa Og* Cag) Iaca@)+aS0Caq) Equation 2: Rate= Egoaion 3: Rate = K[I]" [Sa0^ : ACS20,]. [7 4Cr.] [Bo7] 2At ACI] Bo] %3D 24t Fouation 4: I2 (a2) *2 SəOz*iaq)→cag) + SyO Cag) A[S.0,7 1[3305 Jorig AtSaO,-K [I[B_O/" Equaition 5: Rates - Equation6: Rate:- (rate law) Rate of lass Of reactant SaO"=Alsa05 5.00:10-M These are all of the equations given . II 1. -in order to e ion with perox- y carefully measured va,S,O, solution should be .ette. The KCl and (NH),SO, solu- -crs. Table 3: Initial Rates erefore: **Rate Constant, k **Rate Ra * [S,0,²1mt linit * [Fait init Rate Rxn # can be solved foI 0.0400M 0.0800M data from Rx 0.0400M erature, k sho trations. O.0400M O.0200M 0.0800M 0.0100M carily stain y 0.0800M 4 0.0300M 0.0600M with wate Average value for k Rate Rate Law:
Determine the Rate and Rate Constant CK). Equation 1: 2I'cae) + Sa Og* Cag) Iaca@)+aS0Caq) Equation 2: Rate= Egoaion 3: Rate = K[I]" [Sa0^ : ACS20,]. [7 4Cr.] [Bo7] 2At ACI] Bo] %3D 24t Fouation 4: I2 (a2) *2 SəOz*iaq)→cag) + SyO Cag) A[S.0,7 1[3305 Jorig AtSaO,-K [I[B_O/" Equaition 5: Rates - Equation6: Rate:- (rate law) Rate of lass Of reactant SaO"=Alsa05 5.00:10-M These are all of the equations given . II 1. -in order to e ion with perox- y carefully measured va,S,O, solution should be .ette. The KCl and (NH),SO, solu- -crs. Table 3: Initial Rates erefore: **Rate Constant, k **Rate Ra * [S,0,²1mt linit * [Fait init Rate Rxn # can be solved foI 0.0400M 0.0800M data from Rx 0.0400M erature, k sho trations. O.0400M O.0200M 0.0800M 0.0100M carily stain y 0.0800M 4 0.0300M 0.0600M with wate Average value for k Rate Rate Law:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 36QRT
Related questions
Question
Reaction 1: 47.2 seconds
Reaction 2: 90.2 seconds
Reaction 3: 93.1 seconds
Reaction 4: 196.5 seconds
Reaction 5: 78.4 seconds
![Determine the Rate and Rate Constant CK).
Equation 1: 2I'cae) + Sa Og* Cag) Iaca@)+aS0Caq)
Equation 2: Rate=
Egoaion 3: Rate = K[I]" [Sa0^
: ACS20,]. [7 4Cr.] [Bo7]
2At
ACI] Bo]
%3D
24t
Fouation 4: I2 (a2) *2 SəOz*iaq)→cag) + SyO Cag)
A[S.0,7 1[3305 Jorig
AtSaO,-K [I[B_O/"
Equaition 5: Rates -
Equation6: Rate:-
(rate law)
Rate of lass
Of reactant SaO"=Alsa05 5.00:10-M
These are all of the equations given
.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7df15980-7870-4edc-b87f-7fa0e7a1ef7e%2F87881fba-b3e5-413e-9a9b-ff2bfc926306%2Fctf317.jpeg&w=3840&q=75)
Transcribed Image Text:Determine the Rate and Rate Constant CK).
Equation 1: 2I'cae) + Sa Og* Cag) Iaca@)+aS0Caq)
Equation 2: Rate=
Egoaion 3: Rate = K[I]" [Sa0^
: ACS20,]. [7 4Cr.] [Bo7]
2At
ACI] Bo]
%3D
24t
Fouation 4: I2 (a2) *2 SəOz*iaq)→cag) + SyO Cag)
A[S.0,7 1[3305 Jorig
AtSaO,-K [I[B_O/"
Equaition 5: Rates -
Equation6: Rate:-
(rate law)
Rate of lass
Of reactant SaO"=Alsa05 5.00:10-M
These are all of the equations given
.
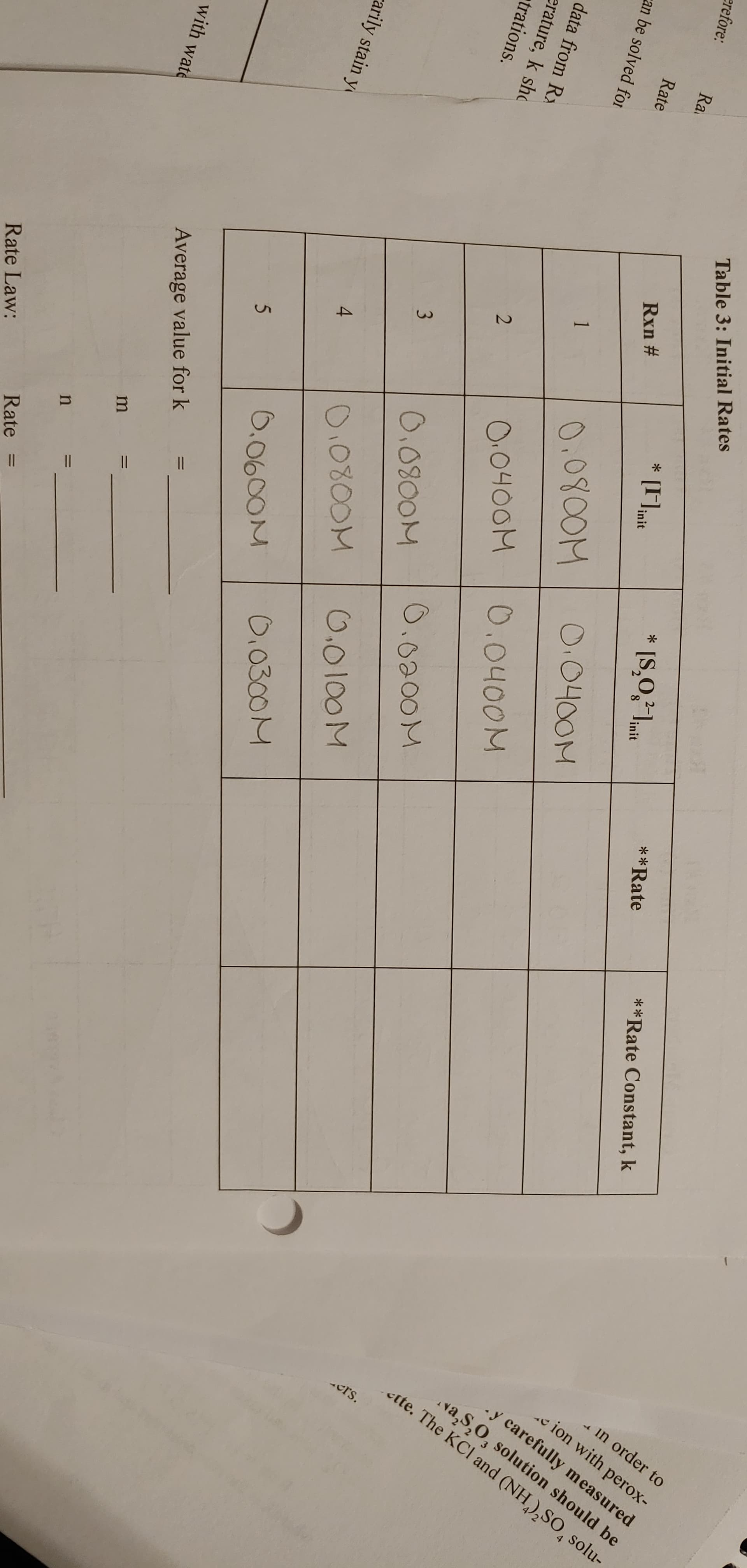
Transcribed Image Text:II
1.
-in order to
e ion with perox-
y carefully measured
va,S,O, solution should be
.ette. The KCl and (NH),SO, solu-
-crs.
Table 3: Initial Rates
erefore:
**Rate Constant, k
**Rate
Ra
* [S,0,²1mt
linit
* [Fait
init
Rate
Rxn #
can be solved foI
0.0400M
0.0800M
data from Rx
0.0400M
erature, k sho
trations.
O.0400M
O.0200M
0.0800M
0.0100M
carily stain y
0.0800M
4
0.0300M
0.0600M
with wate
Average value for k
Rate
Rate Law:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning