Directions: Below is the Concept Map. Fill in the boxes with the word or words that they are connected to each other. Concept words: Sea of electrons Metals Ionic solids Nonpolar Chemical bond Ionic Cations and anions Metallic Covalent Polar Excellent electrical conductors Hard and brittle Low melting point 1. May be 2. 3. 4. Characterized by Consists of May be 5. 6. 7. Exists in Exists in 9. 10. Characterized by ate Characterized by 13. 11. 12. 8.
Directions: Below is the Concept Map. Fill in the boxes with the word or words that they are connected to each other. Concept words: Sea of electrons Metals Ionic solids Nonpolar Chemical bond Ionic Cations and anions Metallic Covalent Polar Excellent electrical conductors Hard and brittle Low melting point 1. May be 2. 3. 4. Characterized by Consists of May be 5. 6. 7. Exists in Exists in 9. 10. Characterized by ate Characterized by 13. 11. 12. 8.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 97AP
Related questions
Question
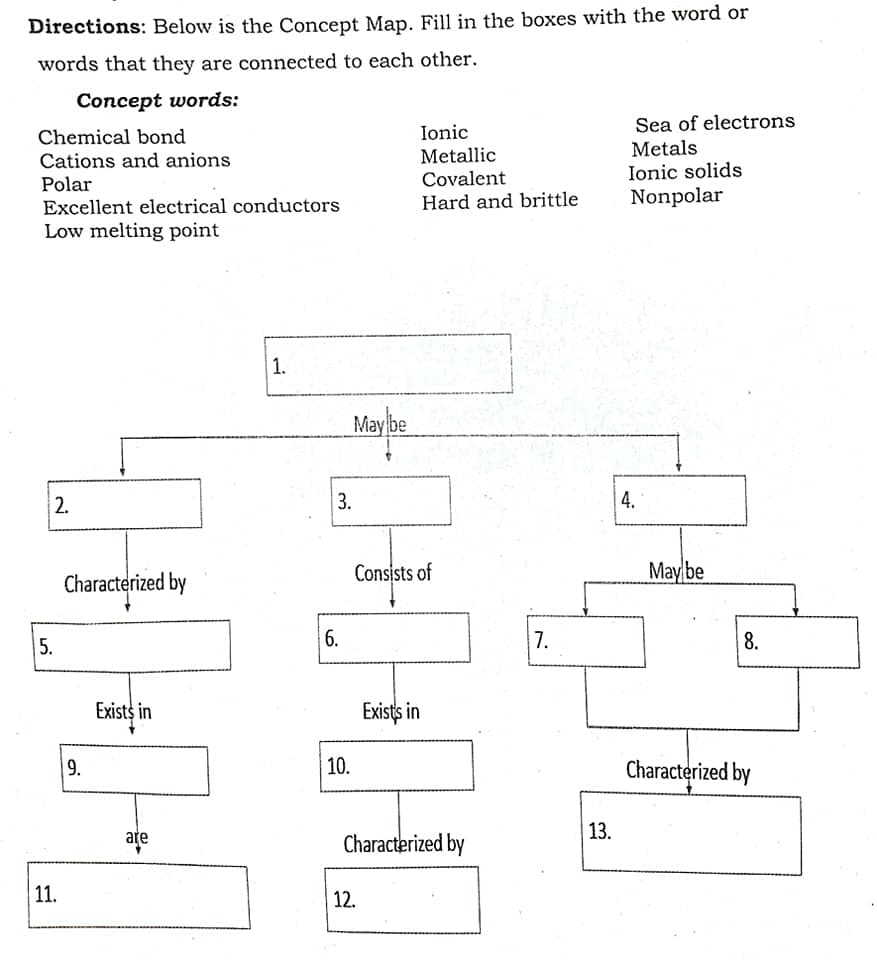
Transcribed Image Text:Directions: Below is the Concept Map. Fill in the boxes with the word or
words that they are connected to each other.
Concept words:
Sea of electrons
Metals
Ionic solids
Nonpolar
Chemical bond
Ionic
Cations and anions
Metallic
Covalent
Polar
Excellent electrical conductors
Low melting point
Hard and brittle
1.
May be
2.
3.
4.
Characterized by
Consists of
May be
5.
7.
6.
8.
Exists in
Exists in
9.
10.
Characterized by
13.
ate
Characterized by
11.
12.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

