Dissolution is an endothermic process when 100 mL of water dissolves 5 grams of a solid compound. Which direction does heat travel during the dissolution? O a. From the system to the surrounding O b. From the surrounding to the system C. Heat does not travel since both the system and the surrounding are at the same final temperatures d. Heat does not travel since both the system and the surrounding are at the same initial temperatures
Dissolution is an endothermic process when 100 mL of water dissolves 5 grams of a solid compound. Which direction does heat travel during the dissolution? O a. From the system to the surrounding O b. From the surrounding to the system C. Heat does not travel since both the system and the surrounding are at the same final temperatures d. Heat does not travel since both the system and the surrounding are at the same initial temperatures
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 7QAP: Magnesium sulfate is often used in first-aid hot packs, giving off heat when dissolved in water. A...
Related questions
Question
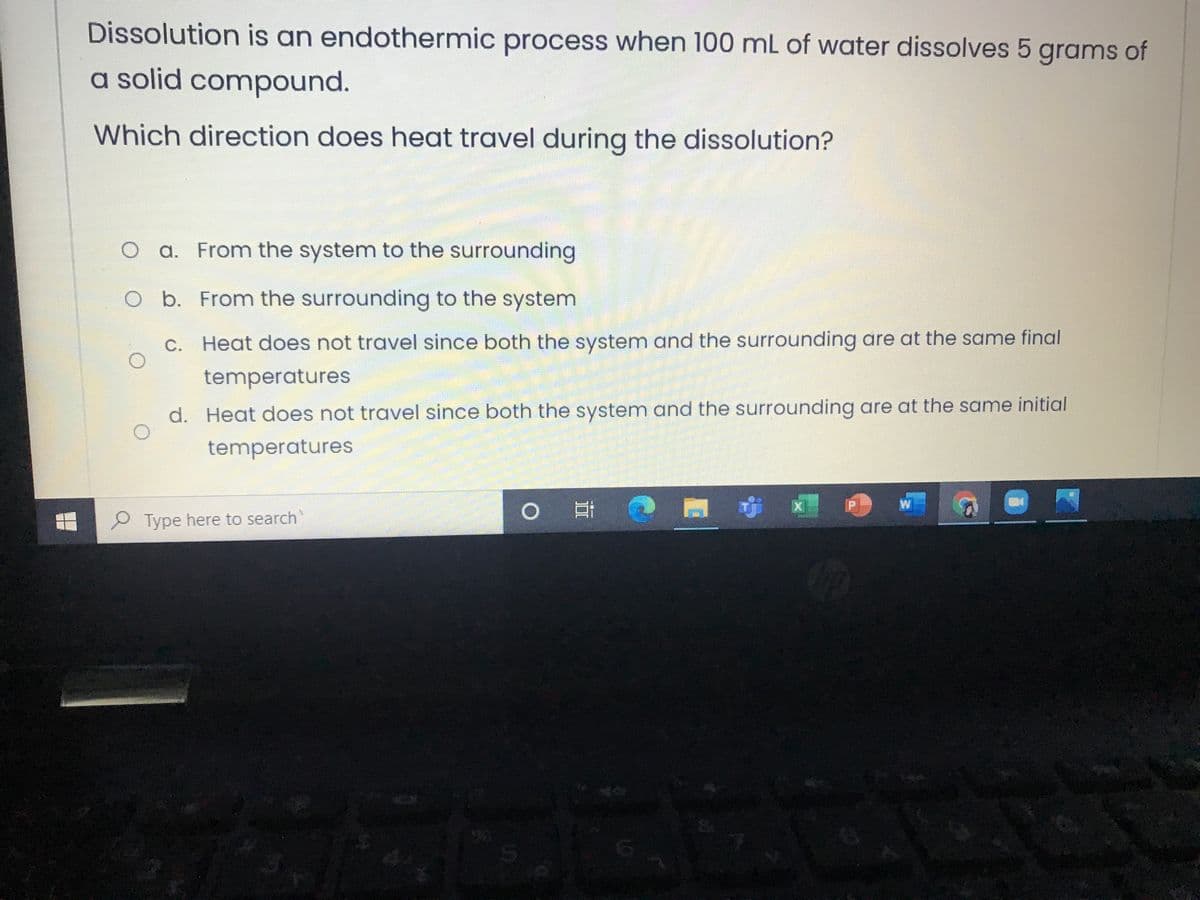
Transcribed Image Text:Dissolution is an endothermic process when 100 mL of water dissolves 5 grams of
a solid compound.
Which direction does heat travel during the dissolution?
a. From the system to the surrounding
O b. From the surrounding to the system
C. Heat does not travel since both the system and the surrounding are at the same final
temperatures
d. Heat does not travel since both the system and the surrounding are at the same initial
temperatures
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning