Distinguish among ionic, covalent, and metallic bonding. Reset Help Each atom in the substance is bonded to several neighboring atoms with bonding electrons relatively free to move throughout the three- dimensional structure. Acquisition of a noble gas electron Metal bonded to Non-Metal configuration by sharing electrons with other atoms. Metal bonded to Metal Acquisition of a noble gas configuration by the transfer of one or more electrons from an atom with a low ionization energy to an atom with a high electron affinity. Non-metal bonded to non-metal lonic Bonding Covalent Bonding Metallic Bonding
Distinguish among ionic, covalent, and metallic bonding. Reset Help Each atom in the substance is bonded to several neighboring atoms with bonding electrons relatively free to move throughout the three- dimensional structure. Acquisition of a noble gas electron Metal bonded to Non-Metal configuration by sharing electrons with other atoms. Metal bonded to Metal Acquisition of a noble gas configuration by the transfer of one or more electrons from an atom with a low ionization energy to an atom with a high electron affinity. Non-metal bonded to non-metal lonic Bonding Covalent Bonding Metallic Bonding
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 67P
Related questions
Question
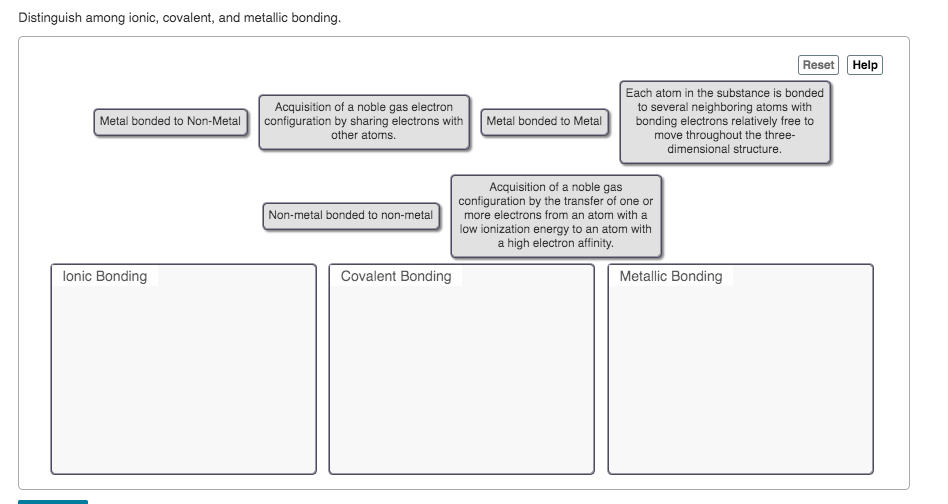
Transcribed Image Text:Distinguish among ionic, covalent, and metallic bonding.
Reset
Help
Each atom in the substance is bonded
to several neighboring atoms with
bonding electrons relatively free to
move throughout the three-
dimensional structure.
Acquisition of a noble gas electron
Metal bonded to Non-Metal
configuration by sharing electrons with
other atoms.
Metal bonded to Metal
Acquisition of a noble gas
configuration by the transfer of one or
more electrons from an atom with a
low ionization energy to an atom with
a high electron affinity.
Non-metal bonded to non-metal
lonic Bonding
Covalent Bonding
Metallic Bonding
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning