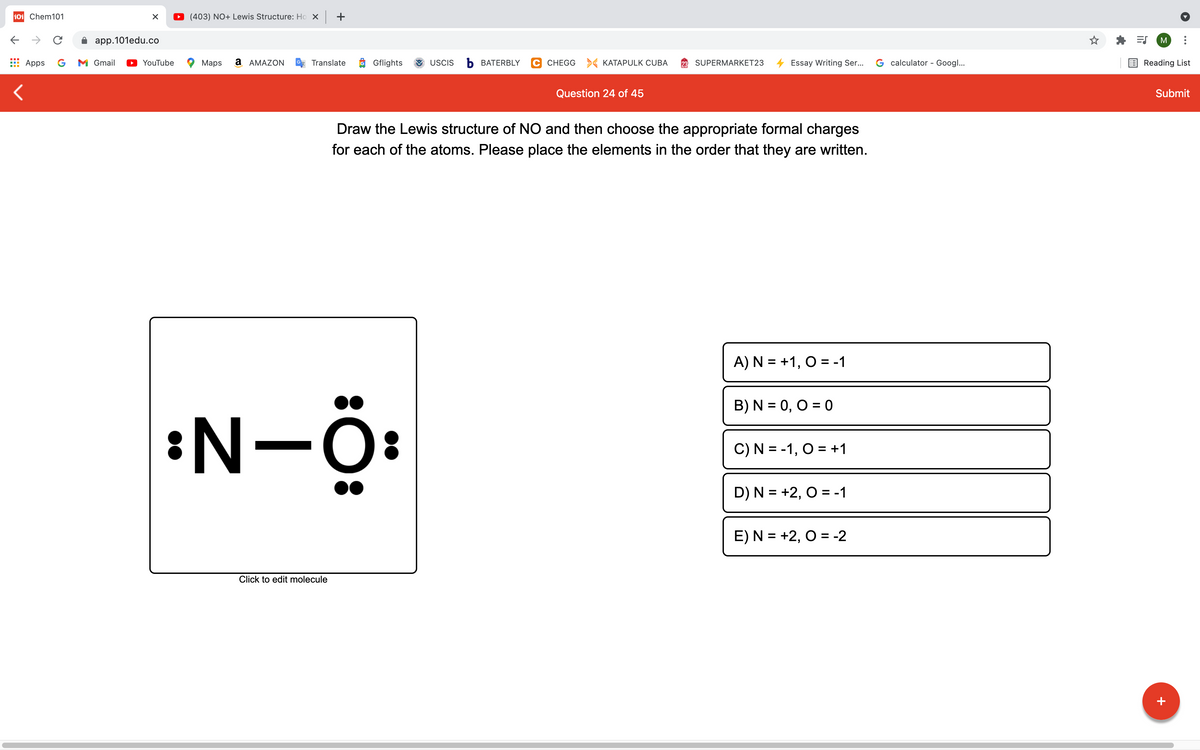
Draw the Lewis structure of NO and then choose the appropriate formal charges for each of the atoms. Please place the elements in the order that they are written. A) N = +1, O = -1 B) N = 0, O = 0 :N-Ö: C) N = -1, O = +1 D) N = +2, O = -1 E)N = +2, 0 = -2 Click to edit molecule
Draw the Lewis structure of NO and then choose the appropriate formal charges for each of the atoms. Please place the elements in the order that they are written. A) N = +1, O = -1 B) N = 0, O = 0 :N-Ö: C) N = -1, O = +1 D) N = +2, O = -1 E)N = +2, 0 = -2 Click to edit molecule
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 94AP: The molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six...
Related questions
Question
Transcribed Image Text:101 Chem101
D (403) NO+ Lewis Structure: Ho X +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
O Gflights
USCIS
ъ ВАТERBLY
C CHEGG KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
Reading List
Question 24 of 45
Submit
Draw the Lewis structure of NO and then choose the appropriate formal charges
for each of the atoms. Please place the elements in the order that they are written.
A)N = +1, O = -1
B) N = 0, O = 0
:N-Ö:
C) N = -1, O = +1
D) N = +2, O = -1
E) N = +2, O = -2
Click to edit molecule
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning