ducing agent, imines NEt NHẸt -H2O EINH, + HC—C—СH, H3C-C-CH3 H, Pt 30 psi ELOH H,C-CH-CH3 (23.22) an imine (not isolated) acetone ethylisopropylamine Reduction of the C=N double bond is analogous to reduction of the C=0 double bond (Sec. 19.8). Notice that thhe imine or enamine does not have to be isolated, but is reduced within the reaction mixture as it forms. Because imines and enamines are reduced more rapidly than carbonyl compounds, reduction of the carbonyl compound is not a competing reaction. PROBLEM 25.14 Provide a reaction mechanism for step 1, formation of the imine, in Eq. 23.22 (Hint: Refer to Sec. 19.11A).
ducing agent, imines NEt NHẸt -H2O EINH, + HC—C—СH, H3C-C-CH3 H, Pt 30 psi ELOH H,C-CH-CH3 (23.22) an imine (not isolated) acetone ethylisopropylamine Reduction of the C=N double bond is analogous to reduction of the C=0 double bond (Sec. 19.8). Notice that thhe imine or enamine does not have to be isolated, but is reduced within the reaction mixture as it forms. Because imines and enamines are reduced more rapidly than carbonyl compounds, reduction of the carbonyl compound is not a competing reaction. PROBLEM 25.14 Provide a reaction mechanism for step 1, formation of the imine, in Eq. 23.22 (Hint: Refer to Sec. 19.11A).
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.51P
Related questions
Concept explainers
Question
23.14

Transcribed Image Text:23.8 HOFMANN ELIMINATION OF QUATERNARY A
12 21 Continue the sequence of reactions in Eqs. 23.16a-c to show how trimethylammonium ioc
in Eq. 23.15.
23,22 Outline a preparation of each of the following from an amine and an acid chloride.
(a) N-phenylbenzamide
(b) N-benzyl-N-ethylpropanamide
23.8
HOFMANN ELIMINATION OF QUATERNARY
AMMONIUM HYDROXIDES
The previous section discussed ways to make carbon-nitrogen bonds. In these reacti
anines react as nucleophiles. The subject of this section is an elimination reaction use
oreak carbon-nitrogen bonds. In this reaction, which involves quaternary ammonium hya
ues (R,N* ¯OH) as starting materials, amines act as leaving groups.
When a quaternary ammonium hydroxide is heated, a B-elimination reaction t.
e to give an alkene, which distills from the reaction mixture.
CH2 NME3
NMe,
-CH, + H-—ОН
heat
trimethylamine
H.
FHO-
a quaternary ammonium
hydroxide
methylenecyclohexane
(74% yield)
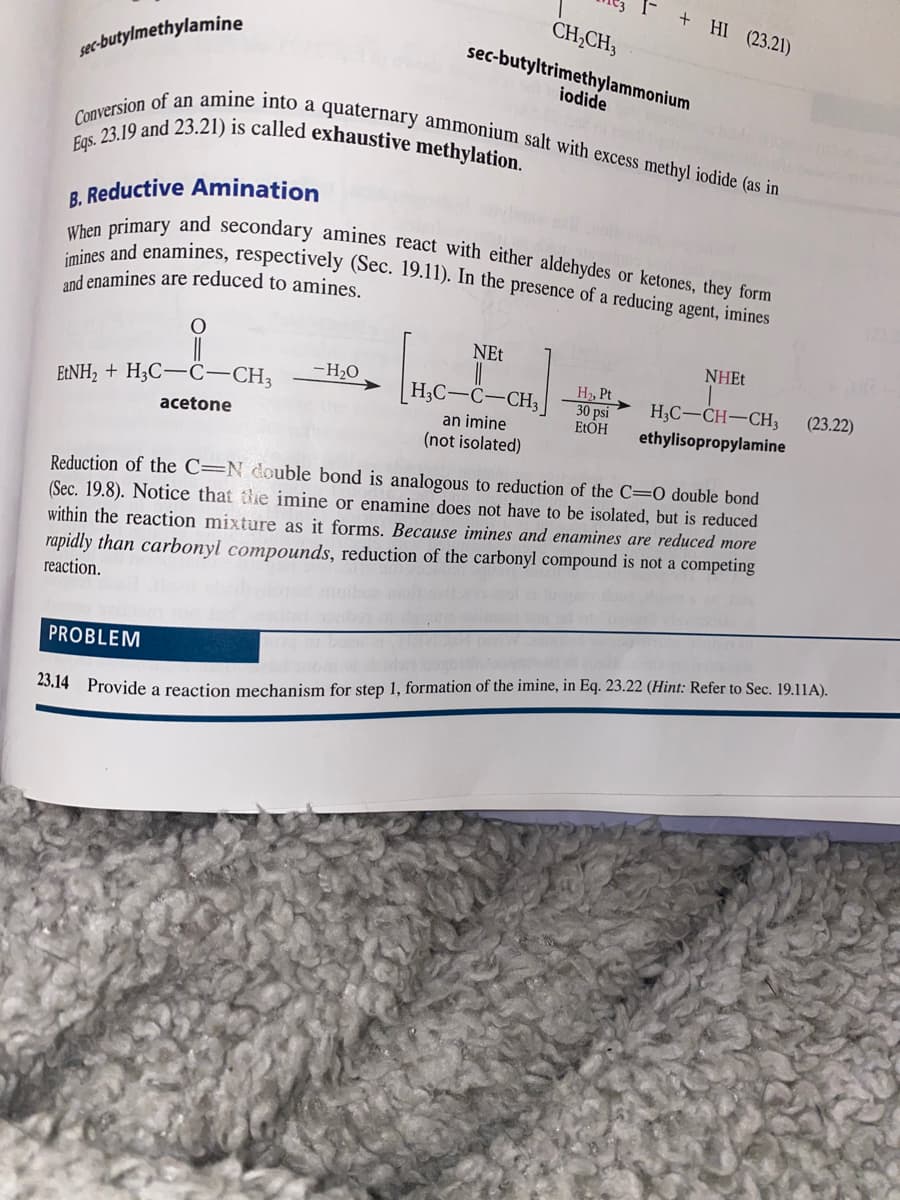
Transcribed Image Text:+ HI (23.21)
Eqs. 23.19 and 23.21) is called exhaustive methylation.
imines and enamines, respectively (Sec. 19.11). In the presence of a reducing agent, imines
When primary and secondary amines react with either aldehydes or ketones, they form
Conversion of an amine into a quaternary ammonium salt with excess methyl iodide (as in
CH;CH3
sec-butyltrimethylammonium
sec-butylmethylamine
iodide
B. Reductive Amination
and enamines are reduced to amines.
NEt
NHE.
-H2O
H2, Pt
30 psi
ELOH
E:NH2 + H3C-C-CH3
H3C-C-CH3
H;C-CH-CH3
(23.22)
an imine
(not isolated)
acetone
ethylisopropylamine
Reduction of the C=N double bond is analogous to reduction of the C=0 double bond
(Sec. 19.8). Notice that te imine or enamine does not have to be isolated, but is reduced
within the reaction mixture as it forms. Because imines and enamines are reduced more
rapidly than carbonyl compounds, reduction of the carbonyl compound is not a competing
reaction.
PROBLEM
23.14 Provide a reaction mechanism for step 1, formation of the imine, in Eq. 23.22 (Hint: Refer to Sec. 19.11A).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole
