(SYN) Which compounds in Problem 23.49 can undergo a Friedel–Crafts reaction? Explain.
When there is an electron-withdrawing group is present on the benzene ring, it cannot goes under the Fridle craft reaction while electron pumping groups actively attached to the benzene ring can promote the Friedel–Crafts reaction. Ortho and para sites of benzene are active sights for Friedel–Crafts reaction.
Go through all options.
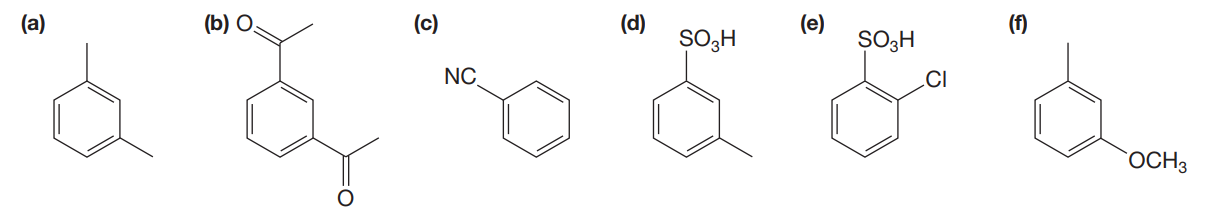
(a) As on the para and ortho position is not suffering from the steric hindrance due to bulky group and electron-donating group -CH3 will promote the Friedel–Crafts reaction.
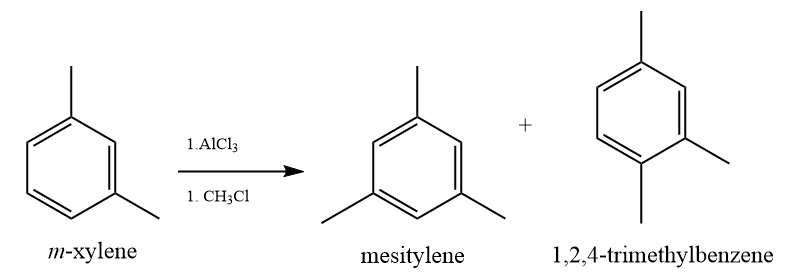
(c) Treatment of benzonitrile with methyl chloride in the presence of Lewis acid aluminum chloride, does not give the product. (-CN) is a strong activating group. It acts as a lewis base too, but it reacts with the Aluminium chloride and attains a positive charge on the nitrogen atom which further deactivates the benzene ring.
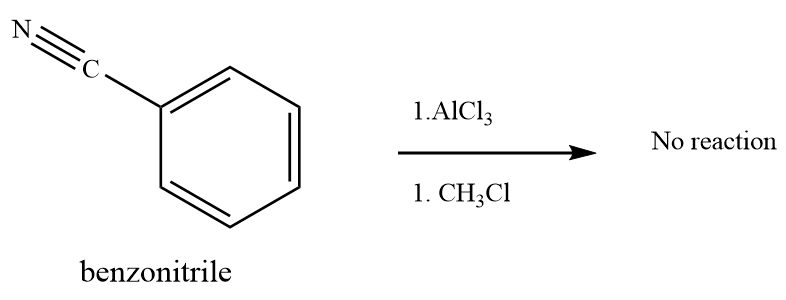
(d) and (e)
Strong deactivating group on benzene ring -SO3H inhibits the Friedel–Crafts reaction.
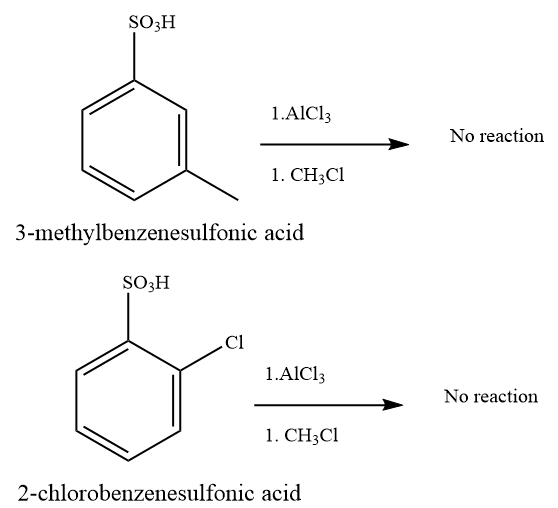
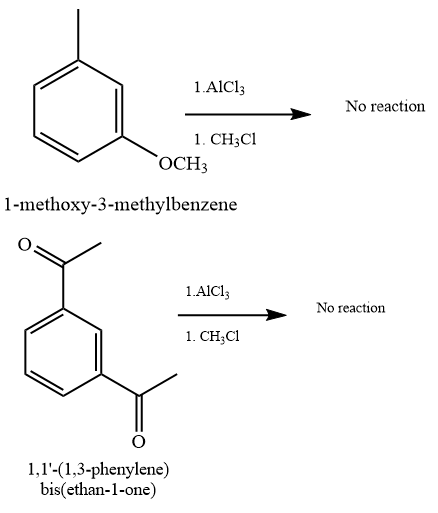
(b) and (f)
Due to steric hindrance, there is no space at the ortho and para position to substitute the new alkyl group. Also, there is an electron-withdrawing effect of oxygen that will deactivate the benzene ring.
Step by step
Solved in 3 steps with 4 images









