Electrolyte Solutions: Milliequivalents TABLE 12.3 VALUES FOR SOME IMPORTANT IONS ATOMIC OR ION FORMULA VALENCE FORMULA WEIGHT EQUIVALENT WEIGHT Al* NH Aluminum 3. 27 6. Ammonium 18 18 Calcium Ca 40 20 Fe*** Fe* Li Ferric 3 56 18.7 Ferrous 2 56 28 Lithium 1 7 Magnesium Potassium Sodium Mg 24 12 K* 1 39 39 23 Na 1 23 Acetate 1 59 59 Bicarbonate Carbonate Chloride HCO5 CO,- 61 61 2 60 30 1 35.5 35.5 Citrate Gluconate CHSO-- 189 63 1 195 195 Lactate CH;O5 H,PO, НРО SO- 1. 89 89 Phosphate 1 97 97 96 48 Sulfate 96 48 Atomic or formula weight Valence Equivalent weight %3D mg mEq x Valence %3D Molecular Weight (MW) mEq x Molecular Weight (MW) mg = Valence mEg x Atomic, formula, or molecular weight mg ml mL Valence Worksheet: 1. What is the concentration, in milligrams per milliliter, of a solution containing 2 mEq of potassium chloride (KCI) per milliliter?
Electrolyte Solutions: Milliequivalents TABLE 12.3 VALUES FOR SOME IMPORTANT IONS ATOMIC OR ION FORMULA VALENCE FORMULA WEIGHT EQUIVALENT WEIGHT Al* NH Aluminum 3. 27 6. Ammonium 18 18 Calcium Ca 40 20 Fe*** Fe* Li Ferric 3 56 18.7 Ferrous 2 56 28 Lithium 1 7 Magnesium Potassium Sodium Mg 24 12 K* 1 39 39 23 Na 1 23 Acetate 1 59 59 Bicarbonate Carbonate Chloride HCO5 CO,- 61 61 2 60 30 1 35.5 35.5 Citrate Gluconate CHSO-- 189 63 1 195 195 Lactate CH;O5 H,PO, НРО SO- 1. 89 89 Phosphate 1 97 97 96 48 Sulfate 96 48 Atomic or formula weight Valence Equivalent weight %3D mg mEq x Valence %3D Molecular Weight (MW) mEq x Molecular Weight (MW) mg = Valence mEg x Atomic, formula, or molecular weight mg ml mL Valence Worksheet: 1. What is the concentration, in milligrams per milliliter, of a solution containing 2 mEq of potassium chloride (KCI) per milliliter?
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.25QAP
Related questions
Question
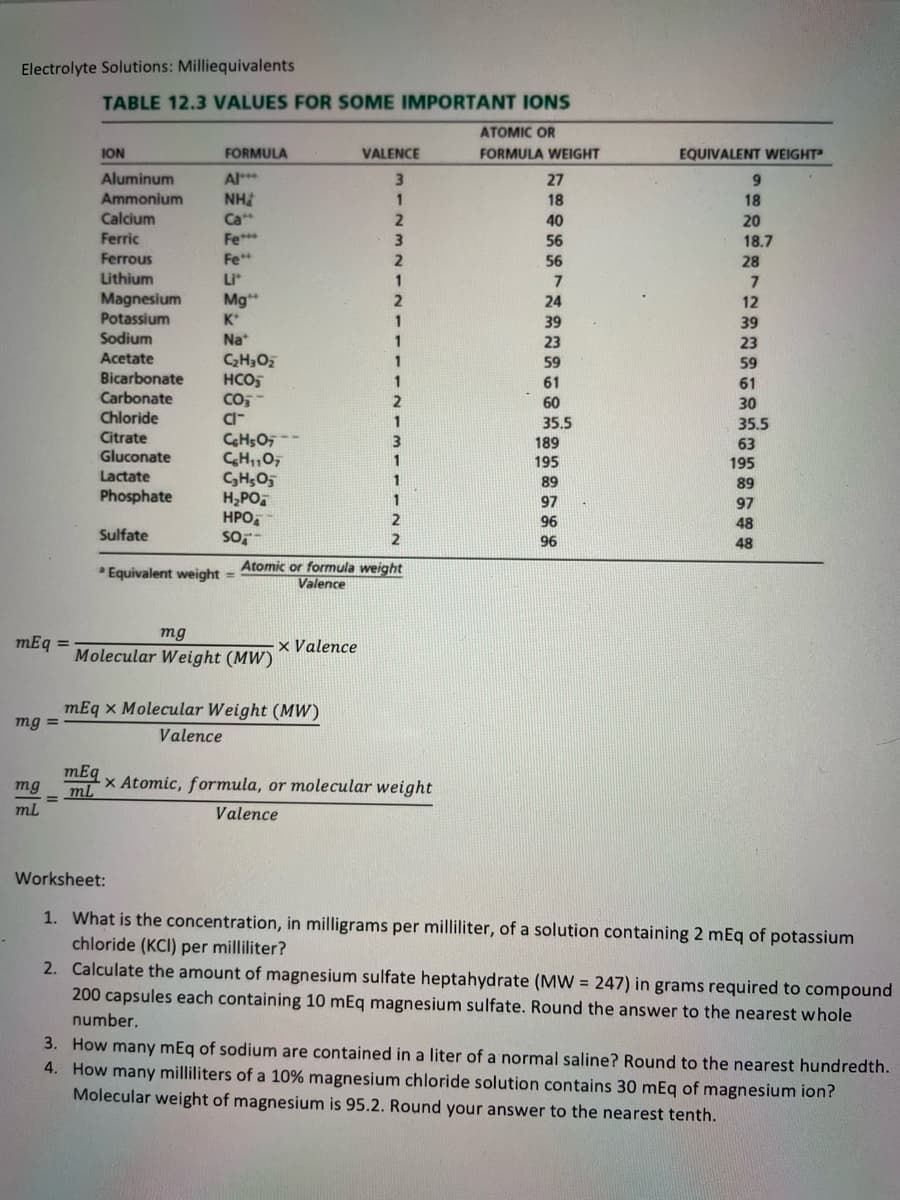
Transcribed Image Text:Electrolyte Solutions: Milliequivalents
TABLE 12.3 VALUES FOR SOME IMPORTANT IONS
ATOMIC OR
ION
FORMULA
VALENCE
FORMULA WEIGHT
EQUIVALENT WEIGHT
Aluminum
Al*
3.
27
9
Ammonium
NH
Ca
1
18
18
Calcium
2.
40
20
Ferric
Fe***
3
56
18.7
Ferrous
Fe*
56
28
Lithium
Magnesium
Potassium
Li
1
7
Mg
24
12
K*
1
39
39
Sodium
Na*
1
23
23
Acetate
1
59
59
Bicarbonate
Carbonate
HCO5
CO,-
1
61
61
60
30
Chloride
1
35.5
35.5
Citrate
189
63
Gluconate
CH,07
CH;O5
H,PO,
НРО
So,-
1
195
195
Lactate
1
89
89
Phosphate
1
97
97
96
48
Sulfate
96
48
Atomic or formula weight
Valence
Equivalent weight =
%3D
mg
mEq =
x Valence
%3D
Molecular Weight (MW)
mEq x Molecular Weight (MW)
mg =
Valence
mEq
mg
ml
x Atomic, formula, or molecular weight
%3D
mL
Valence
Worksheet:
1. What is the concentration, in milligrams per milliliter, of a solution containing 2 mEq of potassium
chloride (KCI) per milliliter?
2. Calculate the amount of magnesium sulfate heptahydrate (MW = 247) in grams required to compound
200 capsules each containing 10 mEq magnesium sulfate. Round the answer to the nearest whole
number.
3. How many mEq of sodium are contained in a liter of a normal saline? Round to the nearest hundredth.
4. How many milliliters of a 10% magnesium chloride solution contains 30 mEq of magnesium ion?
Molecular weight of magnesium is 95.2. Round your answer to the nearest tenth.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning