Electron groups in VSEPR theory can be any of the following: A single unpaired non-bonding electron A lone pair of non-bonding electrons A single bond A doulbe bond A triple bond Select one: O True False Match the following formulas and shapes AX3E Choose... AX4E Choose... AX2E2 Choose...
Electron groups in VSEPR theory can be any of the following: A single unpaired non-bonding electron A lone pair of non-bonding electrons A single bond A doulbe bond A triple bond Select one: O True False Match the following formulas and shapes AX3E Choose... AX4E Choose... AX2E2 Choose...
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 113E: Use the simulation (http://openstaxcollege.org/l/16MolecPolarity) to perform the following exercises...
Related questions
Question
For question 2, shapes include...
1. Bent
2. See-saw
3. Trigonal pyramidal
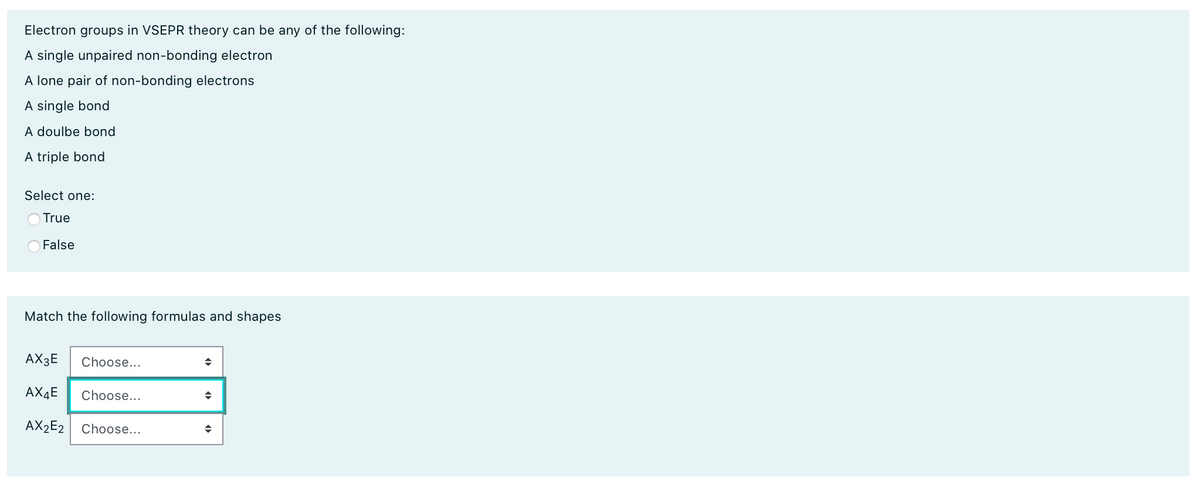
Transcribed Image Text:Electron groups in VSEPR theory can be any of the following:
A single unpaired non-bonding electron
A lone pair of non-bonding electrons
A single bond
A doulbe bond
A triple bond
Select one:
True
False
Match the following formulas and shapes
AX3E
Choose...
AX4E
Choose...
AX2E2
Choose...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning