Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude: fluorine: F2, oxygen: 02, nitrogen: N2 Rank from highest to lowest bond energy. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help nitrogen fluorine охудen Lowest bond energy Highest bond energy The correct ranking cannot be determined.
Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude: fluorine: F2, oxygen: 02, nitrogen: N2 Rank from highest to lowest bond energy. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help nitrogen fluorine охудen Lowest bond energy Highest bond energy The correct ranking cannot be determined.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 76QAP: In each of the following molecules, a central atom is surrounded by a total of three atoms or...
Related questions
Question
100%
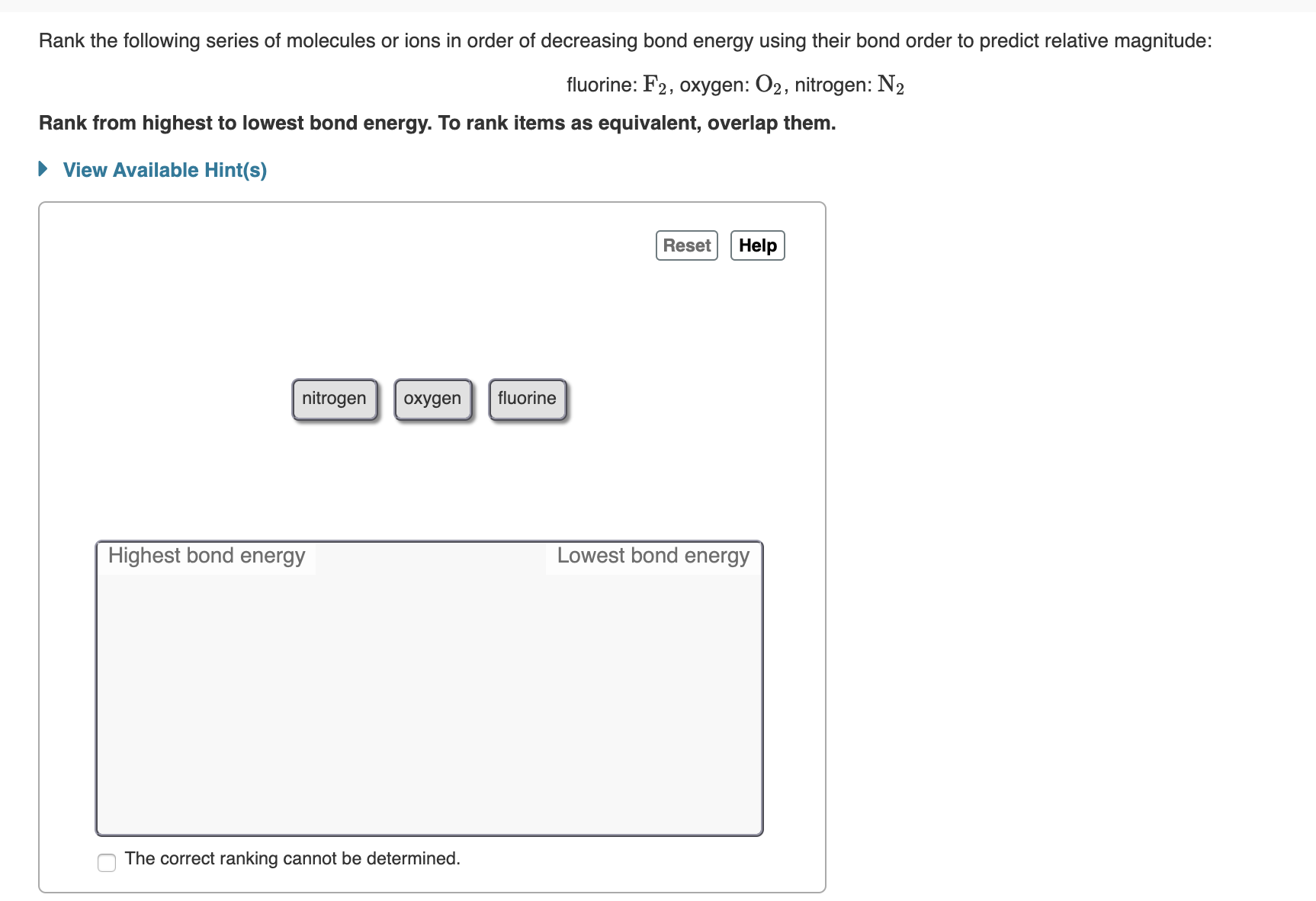
Transcribed Image Text:Rank the following series of molecules or ions in order of decreasing bond energy using their bond order to predict relative magnitude:
fluorine: F2, oxygen: 02, nitrogen: N2
Rank from highest to lowest bond energy. To rank items as equivalent, overlap them.
View Available Hint(s)
Reset
Help
nitrogen
fluorine
охудen
Lowest bond energy
Highest bond energy
The correct ranking cannot be determined.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 6 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning