Element name Group # of Lose or Charg lon Name of Electron And symbol, #3 Valence gain e Formul The ion configuration Atomic # electron electrons on ion Of the lon a Fluorine (F) 9 17 7 Gain 1 -1 F- Flouride 1s(2),25(2),2p(2) ION Magnesium (Mg) 12 Nitrogen (N) 7 2 2 Lost 2 +2 Ng+2 Magnesium 1s(2), 25(2), 2p(6) lon 1s(2), 25(2), 2p(6) 1s(2),25(2).2p(6) 15 Gain 3 -3 N3- Nitride ion Aluminum 13 Lost 3 +3 Al+3 Aluminum (AI)_13 ion Hydrogen (H) Lost 1 +1 H+ Hydrogen 1s(0) 1 ion Making anion Sulfur (S) 16 16 Gain 2 -2 S-2 Sulfur ion 1s(2),25(2),2p(6),3si 2), 3p(6)| Neon (Ne)_10 18 Iron (Fe) *... Lost 2 el. Titanium (TI) 4 ... Lost 3 el. Lead (Pb) 14 ... Lost 4 el. ..... Chromium +3 Co+3
Element name Group # of Lose or Charg lon Name of Electron And symbol, #3 Valence gain e Formul The ion configuration Atomic # electron electrons on ion Of the lon a Fluorine (F) 9 17 7 Gain 1 -1 F- Flouride 1s(2),25(2),2p(2) ION Magnesium (Mg) 12 Nitrogen (N) 7 2 2 Lost 2 +2 Ng+2 Magnesium 1s(2), 25(2), 2p(6) lon 1s(2), 25(2), 2p(6) 1s(2),25(2).2p(6) 15 Gain 3 -3 N3- Nitride ion Aluminum 13 Lost 3 +3 Al+3 Aluminum (AI)_13 ion Hydrogen (H) Lost 1 +1 H+ Hydrogen 1s(0) 1 ion Making anion Sulfur (S) 16 16 Gain 2 -2 S-2 Sulfur ion 1s(2),25(2),2p(6),3si 2), 3p(6)| Neon (Ne)_10 18 Iron (Fe) *... Lost 2 el. Titanium (TI) 4 ... Lost 3 el. Lead (Pb) 14 ... Lost 4 el. ..... Chromium +3 Co+3
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter20: Chemistry Of Hydrogen, Elements In Group 3a Through 6a, And The Noble Gases
Section: Chapter Questions
Problem 20.26QE
Related questions
Question
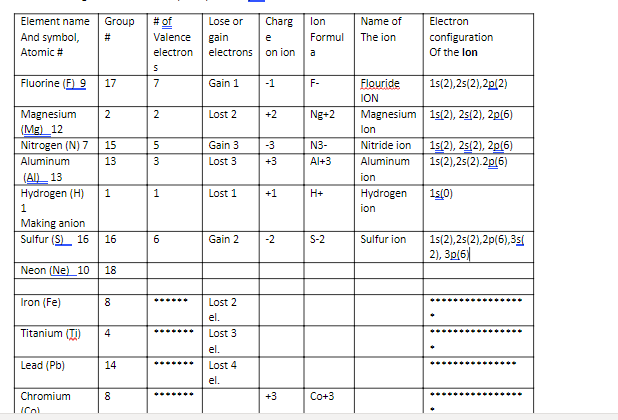
Transcribed Image Text:Element name
Group
# of
Lose or
Charg
lon
Name of
Electron
And symbol,
#3
Valence
gain
e
Formul
The ion
configuration
Atomic #
electron
electrons
on ion
Of the lon
a
Fluorine (F) 9
17
7
Gain 1
-1
F-
Flouride
1s(2),25(2),2p(2)
ION
Magnesium
(Mg) 12
Nitrogen (N) 7
2
2
Lost 2
+2
Ng+2
Magnesium 1s(2), 25(2), 2p(6)
lon
1s(2), 25(2), 2p(6)
1s(2),25(2).2p(6)
15
Gain 3
-3
N3-
Nitride ion
Aluminum
13
Lost 3
+3
Al+3
Aluminum
(AI)_13
ion
Hydrogen (H)
Lost 1
+1
H+
Hydrogen
1s(0)
1
ion
Making anion
Sulfur (S) 16
16
Gain 2
-2
S-2
Sulfur ion
1s(2),25(2),2p(6),3si
2), 3p(6)|
Neon (Ne)_10
18
Iron (Fe)
*...
Lost 2
el.
Titanium (TI)
4
...
Lost 3
el.
Lead (Pb)
14
...
Lost 4
el.
.....
Chromium
+3
Co+3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning