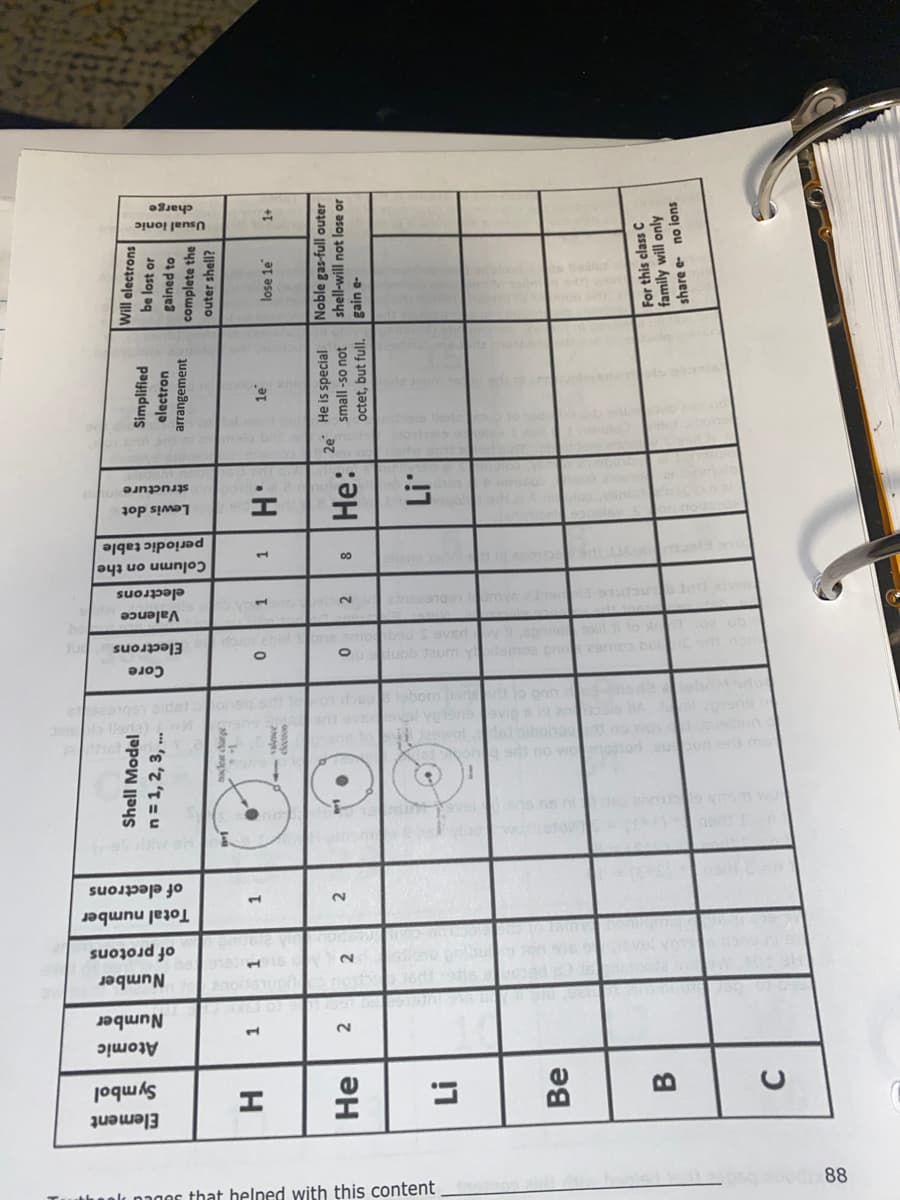
Element Symbol Atomic Number H He Li Be B C 1 2 Number of protons 1 2 Total number of electrons 1 2 Shell Model n = 1, 2, 3, ... : si no worries oborn in jo onn 3-elled2lebo dol Core Electrons 0 Valence electrons gno 29mco bul 1 0 2 2ongs1mge am | Column on the periodic table Lewis dot structure 1 ¹ H 8 He: Li Simplified electron arrangement le 2e He is special small-so not octet, but full. Will electrons be lost or gained to complete the outer shell? lose le Usual ionic charge 1+ Noble gas-full outer shell-will not lose or gain e- For this class C family will only share e- no ions
Element Symbol Atomic Number H He Li Be B C 1 2 Number of protons 1 2 Total number of electrons 1 2 Shell Model n = 1, 2, 3, ... : si no worries oborn in jo onn 3-elled2lebo dol Core Electrons 0 Valence electrons gno 29mco bul 1 0 2 2ongs1mge am | Column on the periodic table Lewis dot structure 1 ¹ H 8 He: Li Simplified electron arrangement le 2e He is special small-so not octet, but full. Will electrons be lost or gained to complete the outer shell? lose le Usual ionic charge 1+ Noble gas-full outer shell-will not lose or gain e- For this class C family will only share e- no ions
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 5P: Estimate the probability of finding an electron which is excited into the 2s orbital of the H atom,...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Transcribed Image Text:al pages that helped with this content
88
Element
Symbol
H
He
Li
Be
B
C
Atomic
Number
2
01
11451
Number
of protons
1
2
Total number
of electrons
1
2
(ve weh
Shell Model
n = 1, 2, 3, ...
OM LUISUA G COLOUR CHE U SU GUS 1566
llada)
er darge
11
si no wo miestod auspun orld mo
329woldat bibohogart no wos
obleas
idat a
GUSLOA (
ET VIL SISCIA SOMAG GUS
COCH LOA
leborn and to poh
topt uger apon Es
Core
Electrons
0
be
owe Suc
Jaum
duob Jeurn yhodomos pro 29mco bicort nor
over by 11,aphild stil 31 to
S
Valence
electrons
LAUK
ratall
1
2
ncpris-Euro Lebusura
por enc
Column on the
periodic table
Lewis dot
structure
1H:
8 He:
Li
Simplified
electron
arrangement
1e
2e He is special
small-so not
octet, but full.
Will electrons
be lost or
gained to
complete the
complete the
outer shell?
lose le
Usual ionic
charge
1+
Noble gas-full outer
shell-will not lose or
gain e-
For this class C
family will only
share e- no ions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning