ELOČOET H2N EtO 'N. H + ELOH Reactions of a primary or secondary amine with diethyl carbonate under controlled conditions gives a carbamic ester. Write a detailed mechanism for this reaction (shown above) which proceeds in 4 steps, including proton transfer steps. Then draw Intermediate 3 in the window provided. The mechanism is detailed as follows: Step 1: Nucleophilic attack to yield zwitterion intermediate 1. Step 2: Protonation/deprotonation (i.e. "proton transfer") of zwitterion 1 to yield intermediate 2. Step 3: Intramolecular collapse of tetrahedral center in intermediate 2 to yield charged intermediate 3. Step 4: Deprotonation of intermediate 3 to yield neutral product. You do not have to consider stereochemistry. In cases where there is more than one answer, just give one. • Do not include counter-ions, e.g., Na", I", in your answer. Do not draw organic or inorganic by-products.
ELOČOET H2N EtO 'N. H + ELOH Reactions of a primary or secondary amine with diethyl carbonate under controlled conditions gives a carbamic ester. Write a detailed mechanism for this reaction (shown above) which proceeds in 4 steps, including proton transfer steps. Then draw Intermediate 3 in the window provided. The mechanism is detailed as follows: Step 1: Nucleophilic attack to yield zwitterion intermediate 1. Step 2: Protonation/deprotonation (i.e. "proton transfer") of zwitterion 1 to yield intermediate 2. Step 3: Intramolecular collapse of tetrahedral center in intermediate 2 to yield charged intermediate 3. Step 4: Deprotonation of intermediate 3 to yield neutral product. You do not have to consider stereochemistry. In cases where there is more than one answer, just give one. • Do not include counter-ions, e.g., Na", I", in your answer. Do not draw organic or inorganic by-products.
Chapter21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
Section21.SE: Something Extra
Problem 36MP: When 4-dimethylaminopyridine (DMAP) is added in catalytic amounts to acetic anhydride and an...
Related questions
Question
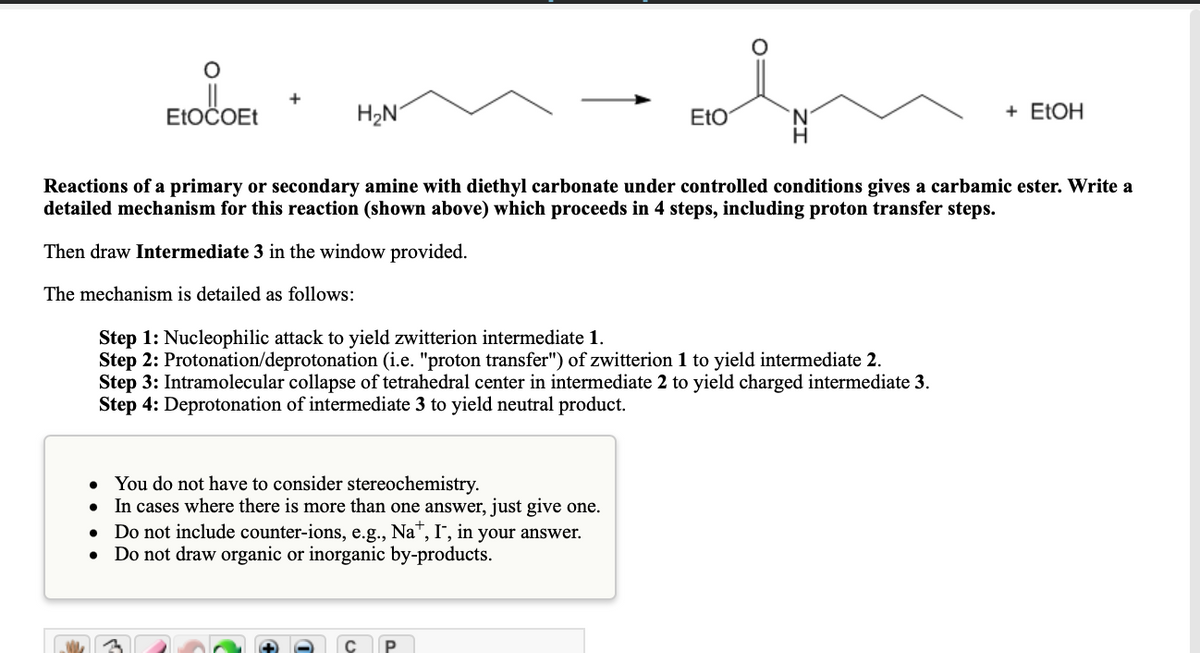
Transcribed Image Text:+
ELOČOET
H2N
EtO
+ ELOH
Reactions of a primary or secondary amine with diethyl carbonate under controlled conditions gives a carbamic ester. Write a
detailed mechanism for this reaction (shown above) which proceeds in 4 steps, including proton transfer steps.
Then draw Intermediate 3 in the window provided.
The mechanism is detailed as follows:
Step 1: Nucleophilic attack to yield zwitterion intermediate 1.
Step 2: Protonation/deprotonation (i.e. "proton transfer") of zwitterion 1 to yield intermediate 2.
Step 3: Intramolecular collapse of tetrahedral center in intermediate 2 to yield charged intermediate 3.
Step 4: Deprotonation of intermediate 3 to yield neutral product.
You do not have to consider stereochemistry.
In cases where there is more than one answer, just give one.
• Do not include counter-ions, e.g., Na",I', in your answer.
Do not draw organic or inorganic by-products.
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning