Enter electrons as e¯. Use smallest possible integer coefficients. If a box is not needed, leave it blank. A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red = 0.337V) and a standard H* | H2 half cell (E°red The anode reaction is: + = 0.000V). + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + + Enter electrons as e. Use smallest possible integer coefficients. If a box is not needed, leave it blank. A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red = 0.337V) and a standard Al3+ | Al half cell (E°red = -1.660V). The anode reaction is: + + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + +
Enter electrons as e¯. Use smallest possible integer coefficients. If a box is not needed, leave it blank. A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red = 0.337V) and a standard H* | H2 half cell (E°red The anode reaction is: + = 0.000V). + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + + Enter electrons as e. Use smallest possible integer coefficients. If a box is not needed, leave it blank. A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red = 0.337V) and a standard Al3+ | Al half cell (E°red = -1.660V). The anode reaction is: + + The cathode reaction is: + The spontaneous cell reaction is: + The cell voltage is V. + +
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question
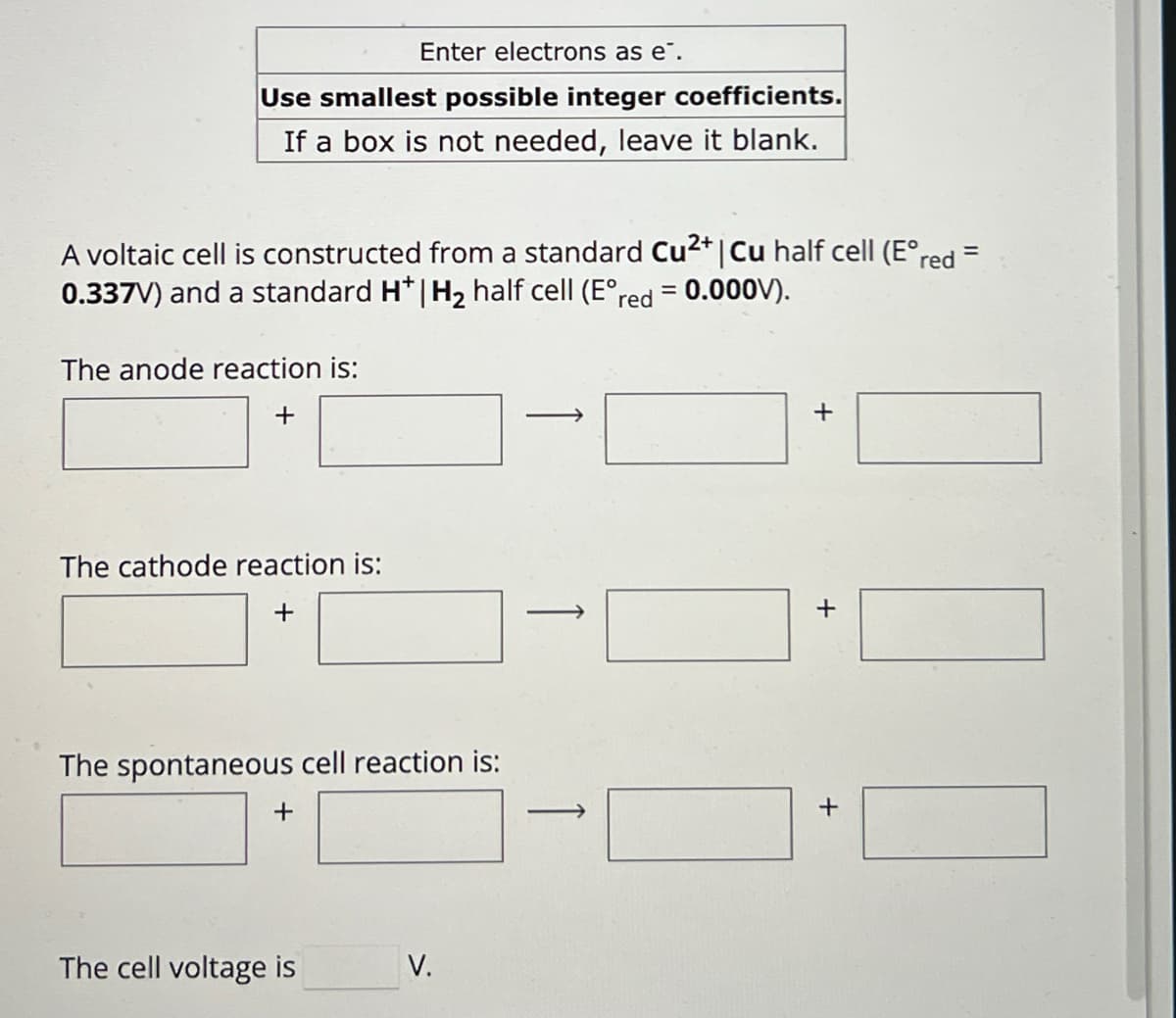
Transcribed Image Text:Enter electrons as e¯.
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.
A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red =
0.337V) and a standard H* | H2 half cell (E°red
The anode reaction is:
+
= 0.000V).
+
The cathode reaction is:
+
The spontaneous cell reaction is:
+
The cell voltage is
V.
+
+
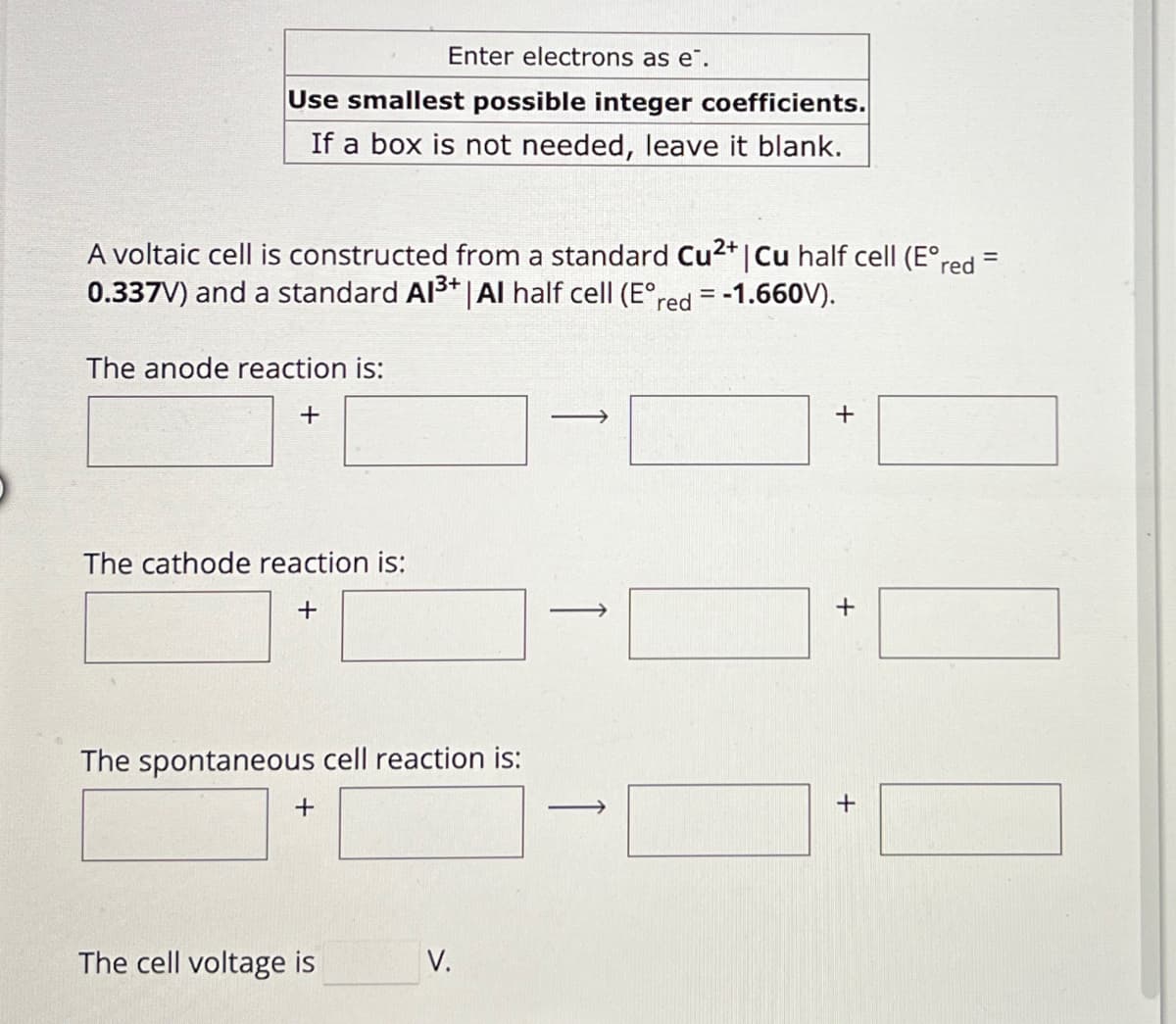
Transcribed Image Text:Enter electrons as e.
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.
A voltaic cell is constructed from a standard Cu2+ | Cu half cell (E°red =
0.337V) and a standard Al3+ | Al half cell (E°red = -1.660V).
The anode reaction is:
+
+
The cathode reaction is:
+
The spontaneous cell reaction is:
+
The cell voltage is
V.
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 18 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning