Enter the noble gas in brackets in the first blank at the top. For the following rows: in the first blank (on the left) in each row, enter the n & subshell. Other blanks after the first one in each row represent the orbital(s) for that subshell. In other blank(s) in each row, enter electrons using u for an up arrow and d for a down arrow. If the orbital should be empty, enter 0 (do not leave it blank). In the final blank, enter the number of unpaired electrons for the element
Enter the noble gas in brackets in the first blank at the top. For the following rows: in the first blank (on the left) in each row, enter the n & subshell. Other blanks after the first one in each row represent the orbital(s) for that subshell. In other blank(s) in each row, enter electrons using u for an up arrow and d for a down arrow. If the orbital should be empty, enter 0 (do not leave it blank). In the final blank, enter the number of unpaired electrons for the element
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter16: Introduction To Magnetic Spectroscopy
Section: Chapter Questions
Problem 16.56E
Related questions
Question
Enter the noble gas in brackets in the first blank at the top.
For the following rows: in the first blank (on the left) in each row, enter the n & subshell.
Other blanks after the first one in each row represent the orbital(s) for that subshell.
In other blank(s) in each row, enter electrons using u for an up arrow and d for a down arrow. If the orbital should be empty, enter 0 (do not leave it blank).
In the final blank, enter the number of unpaired electrons for the element.
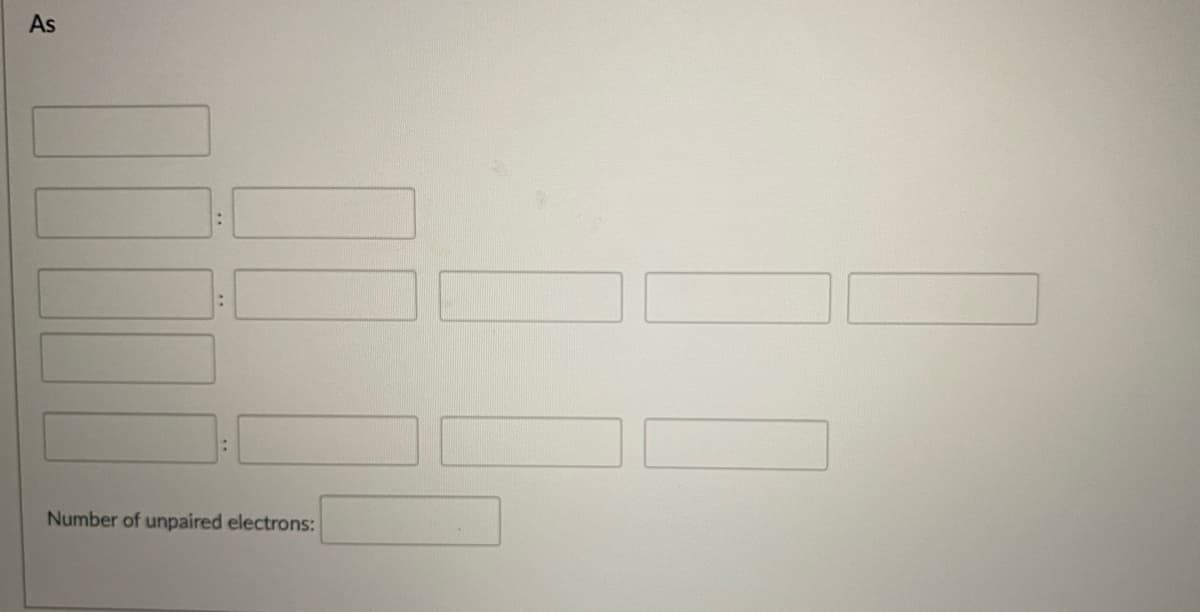
Transcribed Image Text:As
Number of unpaired electrons:
Expert Solution
Step 1
Electronic configuration of an atom is defined as the filling of electrons in increasing order of their subshells. Generally, transition metals show multiple oxidation states. Electronic configuration follows Aufbau principle, Hund’s rule of multiplicity and Pauli’s exclusion principle.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
