Equation #2 P+(CHa), + 2 HCl P+Cl2 + 2CH4 2. c) For equation 2, if 1.0 g of Pt(CH3)2 is reacted with 1.0 g of HCl, what is the quantity of the two products formed (in grams)? Show your work e) Which reactant is the limiting reagent? Why? OWhich reactant is in excess?
Equation #2 P+(CHa), + 2 HCl P+Cl2 + 2CH4 2. c) For equation 2, if 1.0 g of Pt(CH3)2 is reacted with 1.0 g of HCl, what is the quantity of the two products formed (in grams)? Show your work e) Which reactant is the limiting reagent? Why? OWhich reactant is in excess?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section25.8: Applications Of Nuclear Chemistry
Problem 2.3ACP
Related questions
Question
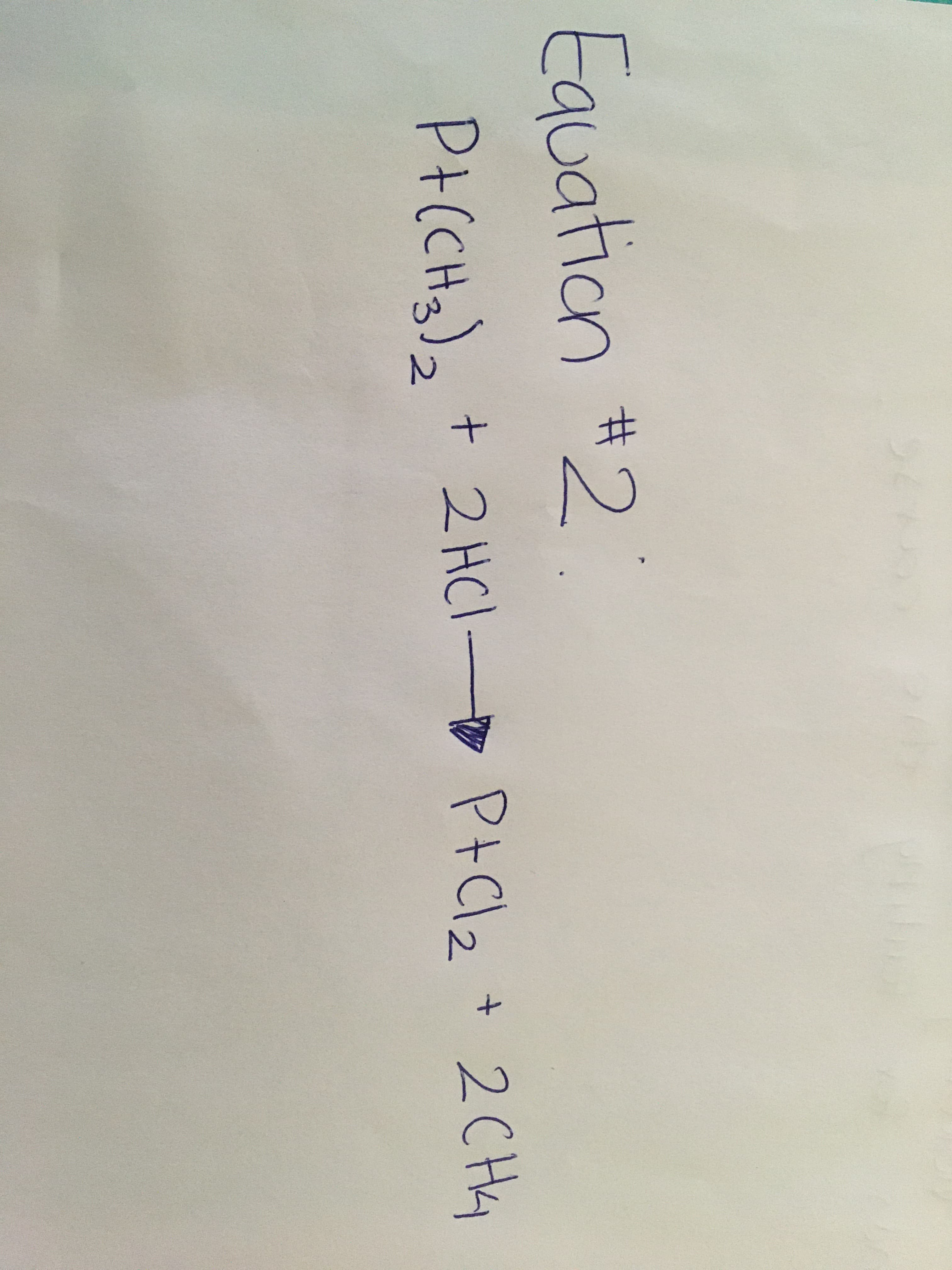
Transcribed Image Text:Equation #2
P+(CHa), + 2 HCl P+Cl2 + 2CH4
2.

Transcribed Image Text:c) For equation 2, if 1.0 g of Pt(CH3)2 is reacted with 1.0 g of HCl, what is the quantity of the
two products formed (in grams)? Show your work
e) Which reactant is the limiting reagent? Why?
OWhich reactant is in excess?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 6 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
