Liquid octane (CH. CH2) CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 4.87 g of water is produced from the reaction of 6.85 g of octane and 15.6 g of oxygen gas, calculate the percent yield of water. Round your answer to 3 significant figures. Continue Submit Assignment 2021 McGraw H LLC A Rigrts Terms of Uhe FriC 0O O D's.20 esc backspac @ #3 6. 8. 1 2 3. 4 W e r tab
Liquid octane (CH. CH2) CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 4.87 g of water is produced from the reaction of 6.85 g of octane and 15.6 g of oxygen gas, calculate the percent yield of water. Round your answer to 3 significant figures. Continue Submit Assignment 2021 McGraw H LLC A Rigrts Terms of Uhe FriC 0O O D's.20 esc backspac @ #3 6. 8. 1 2 3. 4 W e r tab
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 41AP: A dark brown binary compound contains oxygen and ametal. It is 13.38% oxygen by mass. Heating it...
Related questions
Question
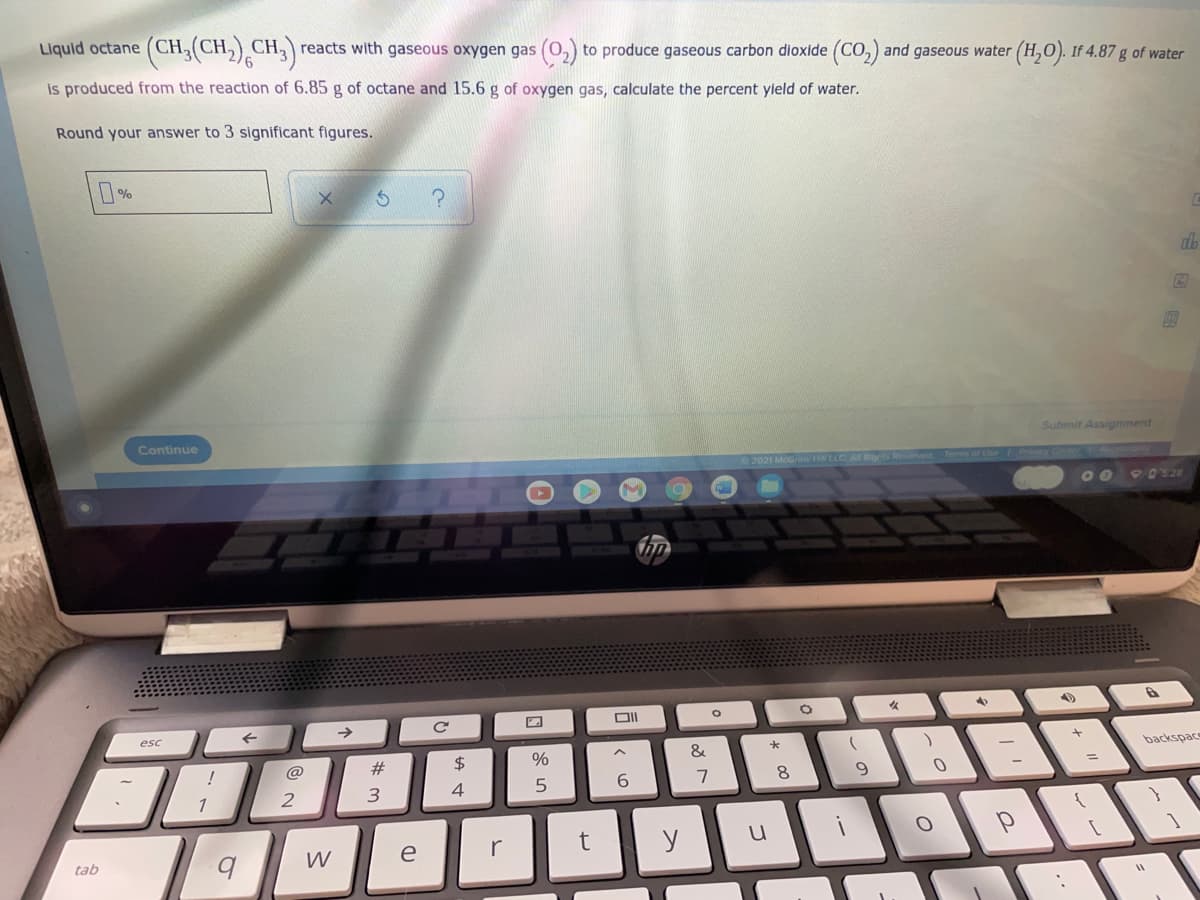
Transcribed Image Text:Liquid octane (CH,(CH,) CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (Co,) and gaseous water (H,O). If 4.87 g of water
6
is produced from the reaction of 6.85 g of octane and 15.6 g of oxygen gas, calculate the percent yield of water.
Round your answer to 3 significant figures.
Continue
Submit Assignment
2021 MCGraw HLLC A Rigts
Terms of Use Privy C
0O O D's.20
esc
backspac
@
#3
$
6.
7
8.
1
2
3.
4
W
e
r
tab
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning