erf z 1.80 0.9891 1.90 0.9928 The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal. The circles represent atoms. Draw its unit cell and show the atoms' positions. To what crystal system does the unit cell belong and what would this crystal structure be called? (10p) 0.30 nm k0.35 nm- 0.40 nm (001) (110) (101) ag az a - az-a3 all angles 900 a -az-azrc angles a3 to c = 900 angles between a axes - 600 a - azc all angles 900 ISOMETRIC HEXAGONAL TETRAGONAL (CUBIC) 口+0k0: a.b.c al angles 900 a.b.c a.b.c all angles A 900 angle between alb and bic - 90 angle between c&a > 90° ORTHORHOMBIC MONOCLINIC TRICLINIC 0.50 nm- 0.46 nm
erf z 1.80 0.9891 1.90 0.9928 The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal. The circles represent atoms. Draw its unit cell and show the atoms' positions. To what crystal system does the unit cell belong and what would this crystal structure be called? (10p) 0.30 nm k0.35 nm- 0.40 nm (001) (110) (101) ag az a - az-a3 all angles 900 a -az-azrc angles a3 to c = 900 angles between a axes - 600 a - azc all angles 900 ISOMETRIC HEXAGONAL TETRAGONAL (CUBIC) 口+0k0: a.b.c al angles 900 a.b.c a.b.c all angles A 900 angle between alb and bic - 90 angle between c&a > 90° ORTHORHOMBIC MONOCLINIC TRICLINIC 0.50 nm- 0.46 nm
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.47E
Related questions
Question
The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal. The circles represent atoms. Draw its unit cell and show the atoms’ positions. To what crystal system does the unit cell belong and what would this crystal structure be called?
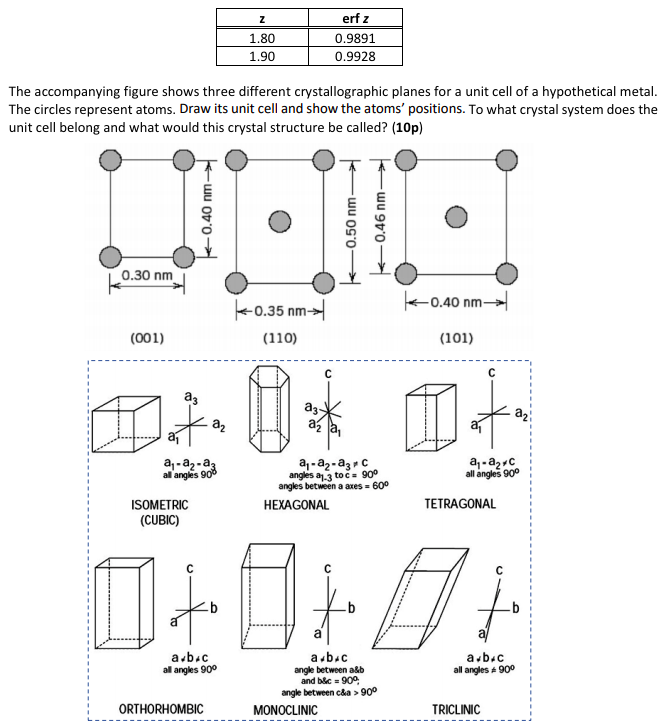
Transcribed Image Text:erf z
1.80
0.9891
1.90
0.9928
The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal.
The circles represent atoms. Draw its unit cell and show the atoms' positions. To what crystal system does the
unit cell belong and what would this crystal structure be called? (10p)
0.30 nm
k0.35 nm-
+0.40 nm-
(001)
(110)
(101)
向 加一
a3
az-
az ja
a - a2-a3
all angles 900
a- a2-az C
angles a.3 to c= 90°
angles between a axes = 600
a, - azrc
all angles 90°
ISOMETRIC
HEXAGONAL
TETRAGONAL
(CUBIC)
C
a
a
a.b.c
all angles 900
a.b.c
angle between a&b
and bac - 900
angle between c&a > 90°
a.b.c
all angles 900
ORTHORHOMBIC
MONOCLINIC
TRICLINIC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning