Ester compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and information in the table to answer these questions: a. Provide a name for each ester that you will prepare. Apple CH;- CH;- CH;-c-ö-CH; b. Draw the structure of each alcohol listed in the table. c. Determine the procedure you will use to prepare each ester, using the reactions found in this chapter. (Hint: Recall that esters are derived from a carboxylic acid and an alcohol.) d. Calculate the cost to prepare 100.0 g of each ester. Which one will be the most expensive to prepare? Which ester will be the least expensive? (Consider only the cost of the alcohols in the table, and disregard the costs of other reagents. Assume 100% yield for all reactions.) Pear CH;-C-0-CH;-CH;-CH, Pineapple CH, —сH,—сH,— €—о-сH,—сн, To prepare these esters, you have been given the alcohols listed in the table and an adequate supply of all other necessary reagents, solvents, and equipment.
Ester compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and information in the table to answer these questions: a. Provide a name for each ester that you will prepare. Apple CH;- CH;- CH;-c-ö-CH; b. Draw the structure of each alcohol listed in the table. c. Determine the procedure you will use to prepare each ester, using the reactions found in this chapter. (Hint: Recall that esters are derived from a carboxylic acid and an alcohol.) d. Calculate the cost to prepare 100.0 g of each ester. Which one will be the most expensive to prepare? Which ester will be the least expensive? (Consider only the cost of the alcohols in the table, and disregard the costs of other reagents. Assume 100% yield for all reactions.) Pear CH;-C-0-CH;-CH;-CH, Pineapple CH, —сH,—сH,— €—о-сH,—сн, To prepare these esters, you have been given the alcohols listed in the table and an adequate supply of all other necessary reagents, solvents, and equipment.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section10.4: Alcohols And Their Oxidation Products
Problem 10.10CE
Related questions
Question
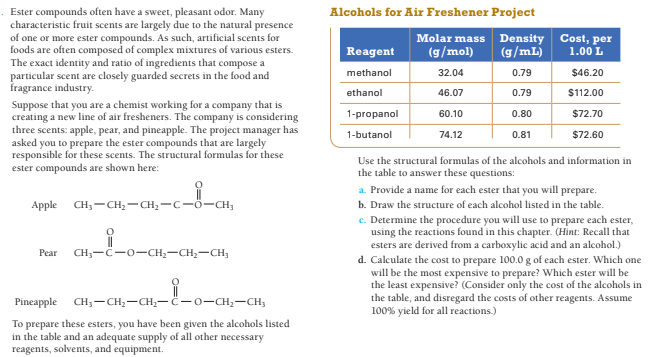
Transcribed Image Text:Ester compounds often have a sweet, pleasant odor. Many
characteristic fruit scents are largely due to the natural presence
of one or more ester compounds. As such, artificial scents for
foods are often composed of complex mixtures of various esters.
The exact identity and ratio of ingredients that compose a
particular scent are closely guarded secrets in the food and
fragrance industry.
Suppose that you are a chemist working for a company that i
creating a new line of air fresheners. The company is considering
three scents: apple, pear, and pineapple. The project manager has
asked you to prepare the ester compounds that are largely
responsible for these scents. The structural formulas for these
ester compounds are shown here:
Alcohols for Air Freshener Project
Molar mass Density Cost, per
(g/mL)
Reagent
(g/mol)
1.00 L
methanol
32.04
0.79
$46.20
ethanol
46.07
0.79
$112.00
1-propanol
60.10
0.80
$72.70
1-butanol
74.12
0.81
$72.60
Use the structural formulas of the alcohols and information in
the table to answer these questions:
a. Provide a name for each ester that you will prepare.
Apple
CH;- CH;- CH;-c-ö-CH;
b. Draw the structure of each alcohol listed in the table.
c. Determine the procedure you will use to prepare each ester,
using the reactions found in this chapter. (Hint: Recall that
esters are derived from a carboxylic acid and an alcohol.)
d. Calculate the cost to prepare 100.0 g of each ester. Which one
will be the most expensive to prepare? Which ester will be
the least expensive? (Consider only the cost of the alcohols in
the table, and disregard the costs of other reagents. Assume
100% yield for all reactions.)
Pear
CH;-C-0-CH;-CH;-CH,
Pineapple CH, —сH,—сH,— €—о-сH,—сн,
To prepare these esters, you have been given the alcohols listed
in the table and an adequate supply of all other necessary
reagents, solvents, and equipment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 8 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning