estion 11 of 39 <> Ernest Rutherford was a prominent scientist who contributed significantly to the theory of atomic structure. Which of the statements are true? Rutherford theorized that atoms contain a positively charged region called the nucleus. Rutherford devised the plum pudding model to describe the layout of an atom. O Rutherford devised the solar system model to describe the layout of the atom. O Rutherford disproved the plum pudding model of the atom.
estion 11 of 39 <> Ernest Rutherford was a prominent scientist who contributed significantly to the theory of atomic structure. Which of the statements are true? Rutherford theorized that atoms contain a positively charged region called the nucleus. Rutherford devised the plum pudding model to describe the layout of an atom. O Rutherford devised the solar system model to describe the layout of the atom. O Rutherford disproved the plum pudding model of the atom.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 16ALQ
Related questions
Question
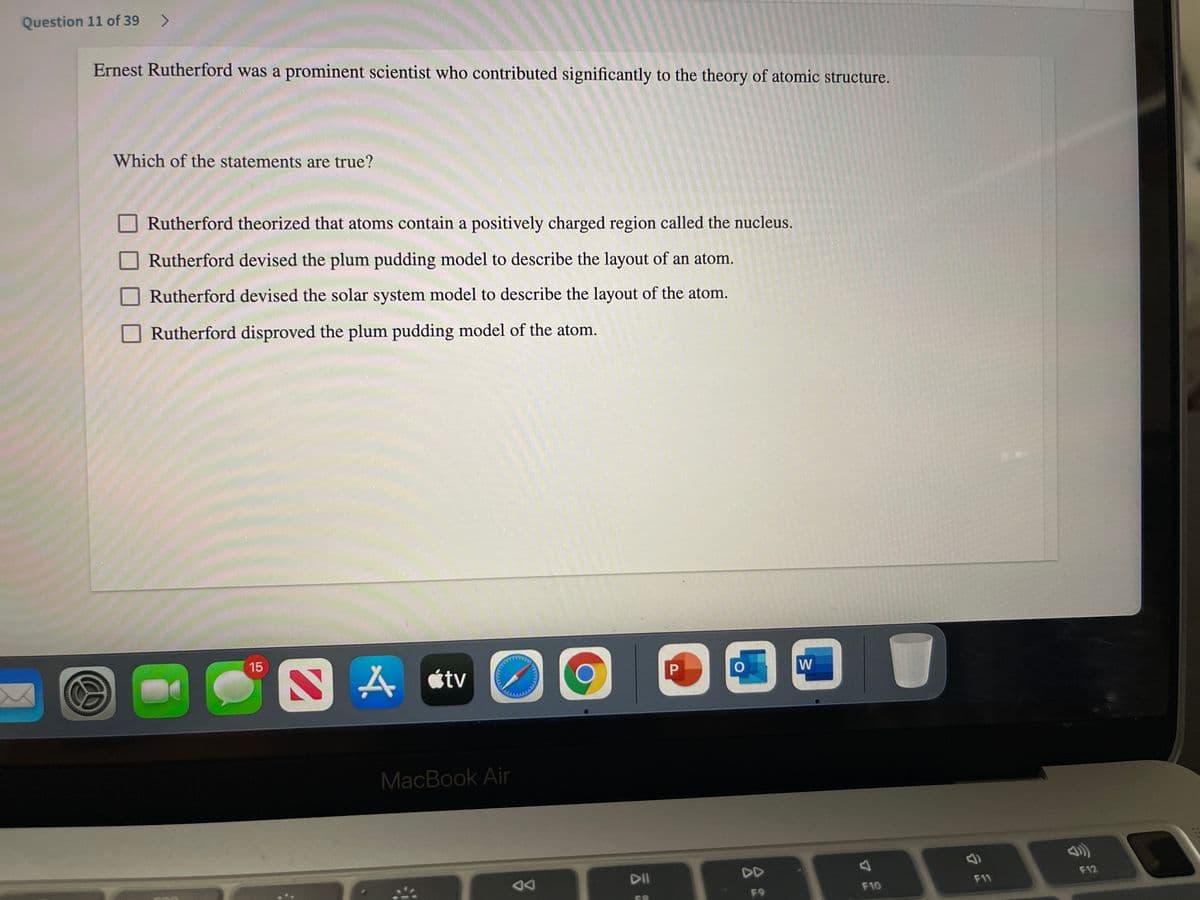
Transcribed Image Text:Question 11 of 39 >
Ernest Rutherford was a prominent scientist who contributed significantly to the theory of atomic structure.
Which of the statements are true?
Rutherford theorized that atoms contain a positively charged region called the nucleus.
Rutherford devised the plum pudding model to describe the layout of an atom.
Rutherford devised the solar system model to describe the layout of the atom.
Rutherford disproved the plum pudding model of the atom.
15
étv
W
MacBook Air
DII
DD
F12
F10
F11
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
