Examine the graph below that was produced when a 12.0 ML sample of acid was titrated with 0.5 M solution of NAOH.calculate the molarity of the acid.
Q: You are asked to make 3.00L solution of sodium chloride with a concentration of 1.30M. What mass of…
A:
Q: Which diagram best represents the result when equal volumes of equal-concentration aqueous solutions…
A: Given : Which diagram best represents the result when equal volumes of equal-concentration aqueous…
Q: is
A: According to the question, we need to identify the correct identification for each statement.
Q: The concentration of a sodium hydroxide solution is to be determined. A 61.76 mL sample of 0.64 M…
A: Given : Concentration of HCl = 0.64 M Volume of HCl sample = 61.76 ml Volume of NaOH = 48.7 ml
Q: During the standardization of NaOH , an air bubble was present in the tip of the buret when making…
A: Standardisation happens by following V1*S1 = V2 * S2 formula, where V1 = volume of standard…
Q: If you have 155 mL solution of a 0.762 M FeCl, solution, how many moles of FeCl, are contained in…
A:
Q: Convert the concentration of 0.700 M Na,SO, to g/mL
A: We use molar mass, which is a gram per mole. Molarity x molar mass = moles/litre x grams /mole the…
Q: Suzy is titrating 10 mL of potassium hydroxide with 6.7 mL of 0.15 M sulfuric acid. What is the…
A: This problem can be solved by using the molarity equation which is : M1 V1n1=M2…
Q: You need to make an aqueous solution of 0.244 M iron(II) iodide for an experiment in lab, using a…
A:
Q: the final volume equals the initial volume of the solution plus the volume of NaOH solution added. ?
A: Given, Molarity of Acid(M) = 0.4500 M Volume of Acid(V) = 78.9 mL mmol of Acid = M × V = 0.4500 ×…
Q: What concentration of Br¯ results when 991 mL of 0.699 M KBr is mixed with 921 mL of 0.527 M FeBr,?…
A: Molarity (M) :- The number of moles of solute dissolved in one litre of solution is defined as…
Q: What NaCl concentration results when 229 mL of a 0.890 M NaCl solution is mixed with 502 mL of a…
A:
Q: Enter your answer in the provided box. Calculate the concentration (in molarity) of an NaOH solution…
A:
Q: Write an equation that will let you calculate the molarity c of this solution. Your equation should…
A: Osmotic pressure The pressure exerted by a solution on the sorrounding system. This pressure is…
Q: How many grams of pure sodium carbonate are needed to standardize a 0.20 N HCl when you expect the…
A: Given that - Normality of HCl = 0.20 N Volume of HCl used = 25.0 mL Then mass of HCl can be…
Q: 3. You need to make 300. mL of a 0.40 M solution of sodium chloride. The only available solution is…
A:
Q: What is the minimum volume, in mL, of a 2.917 M solution of,hydrochloric acid that is required to…
A: The balanced chemical reaction is given by
Q: Enter your answer in the provided box. Determine the resulting nitrate ion concentration when 80.0…
A:
Q: Given a density of 1 g/ml and a molecular weight of 18 g/mol, calculate the concentration of water…
A: Density: It is defined by saying that the ratio of mass to volume. Its units are g/ml. Density=Mass…
Q: What is the final concentration of a solution if 2ml of a 5M solution is added to 5ml of distilled…
A: M1V1 = M2V2 (formula for dilution)
Q: What is concentration of a solution expressed as molarity made by mixing 480 grams of hcl in 600 ml…
A: It is given that :Mass of HCl (solute) = 480 gVolume of solution = 600 mL
Q: Calculate the molarity of a solution of NaCl if 58.6 g of the salt are dissolved in 500.0 ml of…
A: Definition:Molarity is a concentration term for a solution. The molarity of a given solution is…
Q: Determine Whether a Solute Is Soluble in a Solvent?
A: SOLUTION: Step 1: a homogenous mixture of two or more substances in relative amounts that can be…
Q: Does the addition of solvent change the molarity of a solution when a solution is diluted? Explai
A: Molarity(M) is defined as number of moles of solute present in 1000 mL of solution. Molarity=Number…
Q: You need to make an aqueous solution of 0.234 M lead nitrate for an experiment in lab, using a 250…
A: Lead nitrate Solution molarity = 0.234 M = 0.234 mol/L Volume of solution = 250 mL = 0.250 L We…
Q: What is the final concentration of HCl in a solution prepared by addition of 922.0 mL of 4.73 M HCl…
A: Solution stoichiometry involves the calculation of concentration of solutions in the given…
Q: An acid solution was determined to contain 0.022 moles of HCl in a volume of 5.0 mL.Calculate the…
A: Solution stoichiometry involves the calculation of concentration of solutions in the given…
Q: Determine the resulting nitrate ion concentration when 85.0 mL of 0.742 M potassium nitrate and…
A: We need to calculate the molar concentration of nitrate ions in both Potassium nitrate and Calcium…
Q: How would the molarity of acetic acid change if some of the solid oxalic acid was spilled after…
A: Titrimetric method is a useful analytical technique to determine the unknown concentration of a…
Q: Determine the molarity if 0.35 moles of NaCl was dissolved in enough water to make 200. mL of…
A: Molarity of the solution is defined as the number of moles of the solute dissolved in 1 L of the…
Q: If 1.250 moles of NaCl are dissolved in a 250.0 mL solution, what is the concentration of the NaCl…
A:
Q: Suppose you have 350.0 mL of a 0.650 M sodium hydroxide solution. How many moles of sodium hydroxide…
A: 1000ml--->1(M) NaOH sol--->1mol NaOH present or [40 gm(if amount required)]
Q: What mass of sodium carbonate is required to precipitate all of the lead ions from 80.0 mL of 0.100…
A: Lead nitrate reacts with sodium carbonate to form lead carbonate as precipitate. Net Ionic Equation…
Q: If 0.124 moles of NaCl are dissolved in a 250.0 mL solution, what is the concentration of the NaCl…
A:
Q: If you have 155 mL solution of a 0.762 M FeCl, solution, how many grams of FeCl, are contained in…
A: Fill in blanks as shown on problem
Q: Calculate the number of moles of each of the ions in the following solution: 36.4 mL of 0.473 M…
A: Consider the given information is as follows; Volume of Al2SO43 = 36.4 mL = 0.0364 L Concentration…
Q: You are asked to make a 2.50 L solution of sodium iodide with a concentration of 1.80 M. What mass…
A: We have to find the mass of NaO needed
Q: Your manager is confident that you have the skills required to make the solution to assist with her…
A: Given, 500 mL of 0.025 M NaOH molar mass of NaOH = 39.997 g/mol
Q: What concentration of Cl¯ results when 859 mL of 0.519 M LiCl is mixed with 675 mL of 0.299 M MgCl,…
A: Given, Volume of LiCl = 859 mL Concentration of LiCl = 0.519 M Volume of MgCl2 = 675 mL…
Q: Given the following concentration in molar units calculate the concentration in mg/L. 0.066 M H2SO4
A:
Q: Complete and balance and show the complete solution: Manganese Sulfate + Aluminum Phosphate
A: We have to complete and balance and show the complete solution shown below: Manganese Sulfate +…
Q: If 0.050 moles of NaCl are dissolved in a 250.0 mL solution, what is the concentration of the NaCl…
A: Given data, Number of moles of NaCl = 0.050 mol Volume of the solution = 250 ml 1 L = 1000 ml…
Q: Create a data table with the following columns: Column 1 Title: Molarity of Vinegar (Record this as…
A: Data table for the above given is
Q: Compute the volume in mL of a 0.1552 M solution of NaOH required to exactly react with a 0.5284g…
A: Given: Mass of KHP = 0.5284 g And concentration of NaOH = 0.1552 M
Q: If 0.400 moles of NaCl are dissolved in a 250.0 mL solution, what is the concentration of the NaCl…
A: Concentration in terms of molarity is defined as the moles of solute present per litre of the…
Q: Calculate the concentration (M) of calcium and phosphate ions when 139 grams of calcium phosphate is…
A: We have 139 gram of calcium phosphate dissolved in water to form 297.88 mL of Solution. We have to…
Q: The mass of manganese(II) hydroxide that is dissolved in 125 mL of a saturated solution is _______…
A:
Q: An aqueous solution of calcium hydroxide is standardized by titration with a 0.100 M solution of…
A: Molarity of HCl = 0.1 M Volume of acid = 21 mL Volume of base = 22.8 mL
Q: If 0.166 moles of NaCl are dissolved in a 250.0 mL solution, what is the concentration of the NaCl…
A: No. of moles of NaCl = 0.166 Volume of solution = 250 ml Now we have to apply the concentration…
Step by step
Solved in 2 steps with 2 images

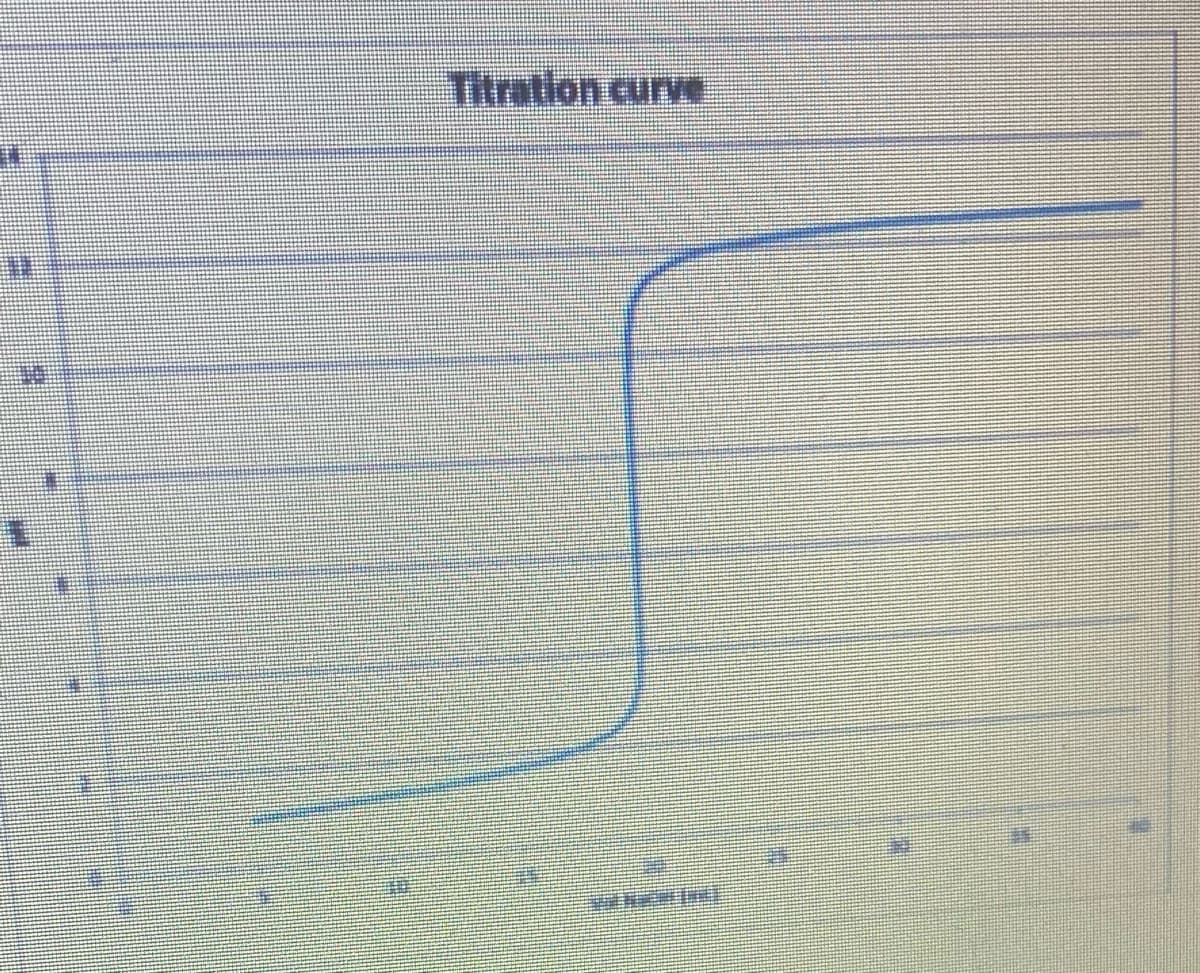
- What happens to the calculated molarity of the sample if the burette was not washed with the standardized titrant prior to titration? Increase Decrease No Effect IndeterminateAcid Based Titration QuestionConstruct a titration curve from the data below. Determine the volume of titrant at equivalence point.
- Show excel table of values for the titration curve of the given problem. An example is attached as a reference for the table of values from Excel.How titration technique can be used to determine the concentration of acetic acid in vinegar sample? (3 sentences)why are equivalence points on a titration graph equally spaced along the horizontal axis?

