Express your answer in atomic mass units to four significant figures. atomic mass of Rb amu Submit Request Answer Part D Silcon has three naturally occurring isotopes: Si-28 with mass 27.9769 amu and a natural abundance of 92.21%, Si-29 with mass 2 natural abundance of 4.69%, and Si-30 with mass 29.9737 amu and a natural abundance of 3.10%. Calculate the atomic mass of sili Express your answer in atomic mass units to four significant figures. ?
Express your answer in atomic mass units to four significant figures. atomic mass of Rb amu Submit Request Answer Part D Silcon has three naturally occurring isotopes: Si-28 with mass 27.9769 amu and a natural abundance of 92.21%, Si-29 with mass 2 natural abundance of 4.69%, and Si-30 with mass 29.9737 amu and a natural abundance of 3.10%. Calculate the atomic mass of sili Express your answer in atomic mass units to four significant figures. ?
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 11STP
Related questions
Question
100%
Practice Pack
Answer both questions please
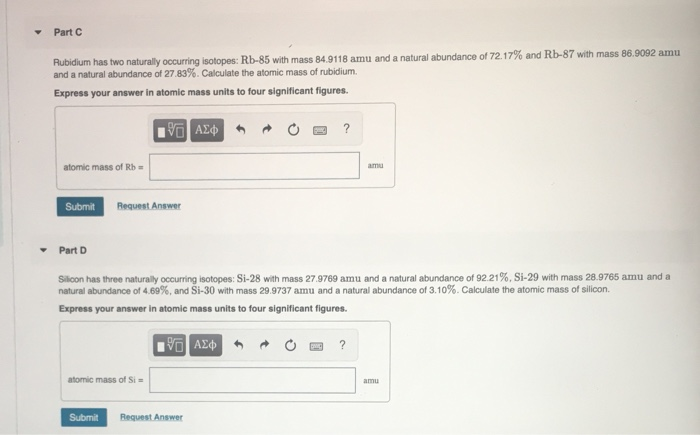
Transcribed Image Text:Part C
Rubidium has two naturally occurring isotopes: Rb-85 with mass 84.9118 amu and a natural abundance of 72.17% and Rb-87 with mass 86.9092 amu
and a natural abundance of 27.83%. Calculate the atomic mass of rubidium.
Express your answer in atomic mass units to four significant figures.
?
atomic mass of Rb =
amu
Submit
Request Anewer
Part D
Silicon has three naturally occurring isotopes: Si-28 with mass 27.9769 amu and a natural abundance of 92.21%, Si-29 with mass 28.9765 amu and a
natural abundance of 4.69%, and Si-30 with mass 29.9737 amu and a natural abundance of 3.10%. Calculate the atomic mass of silicon.
Express your answer in atomic mass units to four significant figures.
atomic mass of Si =
amu
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Includes step-by-step video
Trending now
This is a popular solution!
Learn your way
Includes step-by-step video
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER
