(f) A certain pure compound containing element E is analyzed. The elemental composition of the compound is shown in the following table. Determine the empirical formula of the compound using the information in the table. Element Moles Present in the Compound E 0.0676 0.0338 0.102 в IU х? х, Ω III !!
(f) A certain pure compound containing element E is analyzed. The elemental composition of the compound is shown in the following table. Determine the empirical formula of the compound using the information in the table. Element Moles Present in the Compound E 0.0676 0.0338 0.102 в IU х? х, Ω III !!
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.161QP
Related questions
Question
Need help with these question
![Initial [HBr (aq)]
Volume of H2 (g) Produced
Trial
Initial Moles of E (s)
(М)
(mL)
1
0.00220
0.500
27.02
0.00240
0.500
29.40
3
0.00280
0.500
?
(e) The data from all three trials are given above. A student claims that HBr (aq) is the limiting reactant in trial 1. Do you agree or disagree? Justify your answer
with information in the data table.
I
x? X, 5
Ω
Bold
0 / 10000 Word Limit
(f) A certain pure compound containing element E is analyzed The elemental composition of the compOund is shown in the following table Determine the empirical
!!!
B](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Feb95b132-2b3c-45c1-919c-6cbd19c0a0e7%2F6cf50dca-b4ab-4030-8326-616323442ff1%2F0yzn0u9_processed.png&w=3840&q=75)
Transcribed Image Text:Initial [HBr (aq)]
Volume of H2 (g) Produced
Trial
Initial Moles of E (s)
(М)
(mL)
1
0.00220
0.500
27.02
0.00240
0.500
29.40
3
0.00280
0.500
?
(e) The data from all three trials are given above. A student claims that HBr (aq) is the limiting reactant in trial 1. Do you agree or disagree? Justify your answer
with information in the data table.
I
x? X, 5
Ω
Bold
0 / 10000 Word Limit
(f) A certain pure compound containing element E is analyzed The elemental composition of the compOund is shown in the following table Determine the empirical
!!!
B
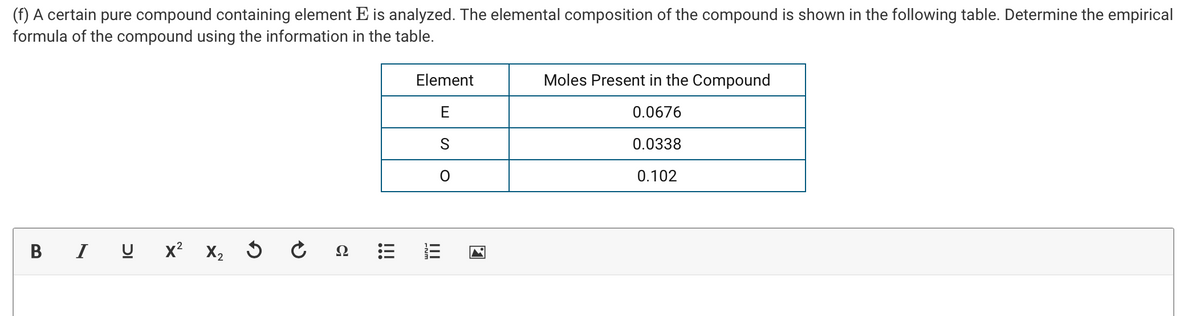
Transcribed Image Text:(f) A certain pure compound containing element E is analyzed. The elemental composition of the compound is shown in the following table. Determine the empirical
formula of the compound using the information in the table.
Element
Moles Present in the Compound
E
0.0676
0.0338
0.102
В
I U x? X, 5
II
!!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning