File Edit View History Bookmarks Profiles Tab Window Help * O D $ D Fri Sep 17 ••• I Course Home HOCH2CH2CH2NH2 conjugate x Predict the products of the acic x + i openvellum.ecollege.com/course.html?courseld=16921657&OpenVellumHMAC=3e9d806a764c710a5b0109e6b2381d4f#10001 E YouTube Netflix & Khan D Turnitin Zoom E Canvas P Pearson Sign In | Reading List partleby Mastering Chemistry Course Home O My Courses
File Edit View History Bookmarks Profiles Tab Window Help * O D $ D Fri Sep 17 ••• I Course Home HOCH2CH2CH2NH2 conjugate x Predict the products of the acic x + i openvellum.ecollege.com/course.html?courseld=16921657&OpenVellumHMAC=3e9d806a764c710a5b0109e6b2381d4f#10001 E YouTube Netflix & Khan D Turnitin Zoom E Canvas P Pearson Sign In | Reading List partleby Mastering Chemistry Course Home O My Courses
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.77PAE
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
Fri Sep 17 0:17
Course Home
G HOCH2CH2CH2NH2 conjugate x
Predict the products of the aci X
+
openvellum.ecollege.com/course.html?courseld=16921657&OpenVellumHMAC=3e9d806a764c710a5b0109e6b2381d4f#10001
O < >
YouTube
Netflix
Khan
Turnitin
O Zoom
N Canvas
Pearson Sign In
E Reading List
Course Home
= bartleby Mastering Chemistry
Мy Courses
<Assignment 2
e Q&A Library
Course Home
Problem 2.61 (a) - Enhanced - with Feedback
7 of 10
>
Syllabus
Question
Part A
Scores
ar
Draw the structur
eТext
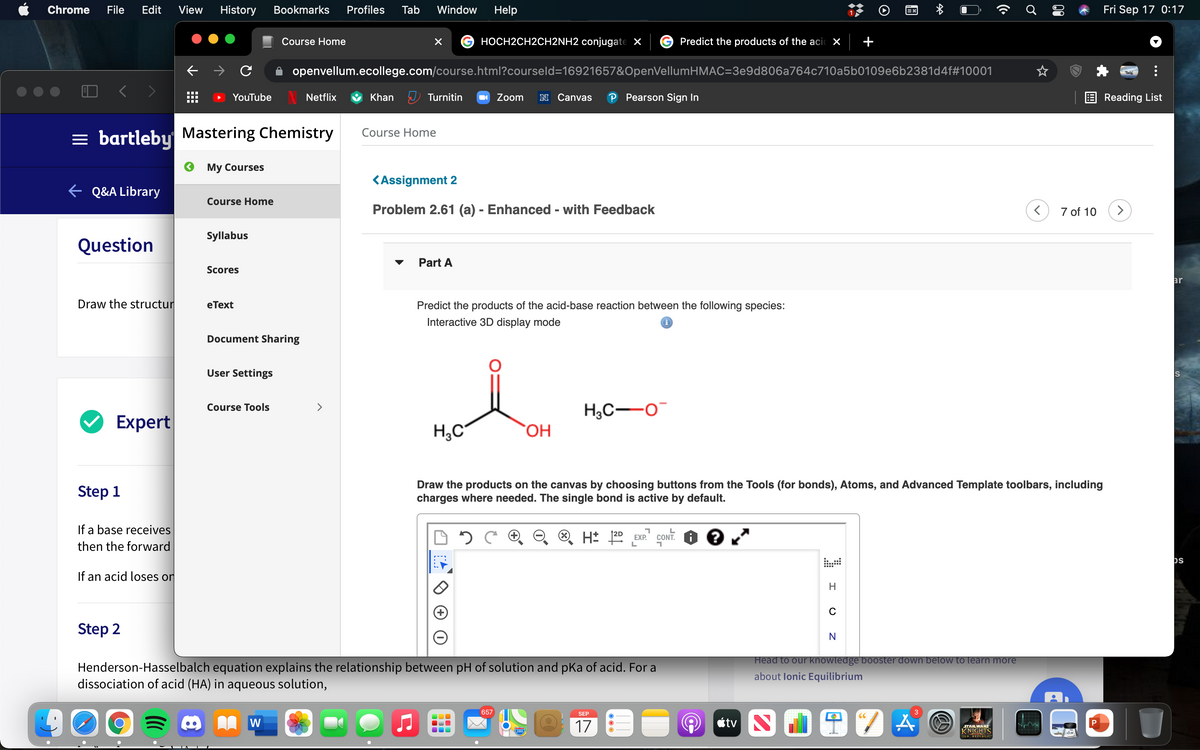
Predict the products of the acid-base reaction between the following species:
Interactive 3D display mode
Document Sharing
User Settings
Course Tools
<>
H3C-O
Expert
H;C
Step 1
Draw the products on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including
charges where needed. The single bond is active by default.
If a base receives
O 5 C ® Q ® H# 2 EXP.
L
CONT.
then the forward
If an acid loses or
H
Step 2
Head to our knowledge booster down below to learn more
Henderson-Hasselbalch equation explains the relationship between pH of solution and pKa of acid. For a
dissociation of acid (HA) in aqueous solution,
about lonic Equilibrium
657
SEP
3
CC
W
17
étv
STARWARS
KNIGHTS
OLD REPUBLIC
280
圖
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,