Find the concentration of I- in 0.05 M AgNO3 saturated with AgI. Include activity coeffcients in the solubility-product expression. The Ksp of AgI is 8.3x10-17. Refer to the table of activity coefficients at various ionic strengths as needed.
Find the concentration of I- in 0.05 M AgNO3 saturated with AgI. Include activity coeffcients in the solubility-product expression. The Ksp of AgI is 8.3x10-17. Refer to the table of activity coefficients at various ionic strengths as needed.
Chapter34: Miscellaneous Separation Methods
Section: Chapter Questions
Problem 34.19QAP
Related questions
Question
Find the concentration of I- in 0.05 M AgNO3 saturated with AgI. Include activity coeffcients in the solubility-product expression. The Ksp of AgI is 8.3x10-17. Refer to the table of activity coefficients at various ionic strengths as needed.
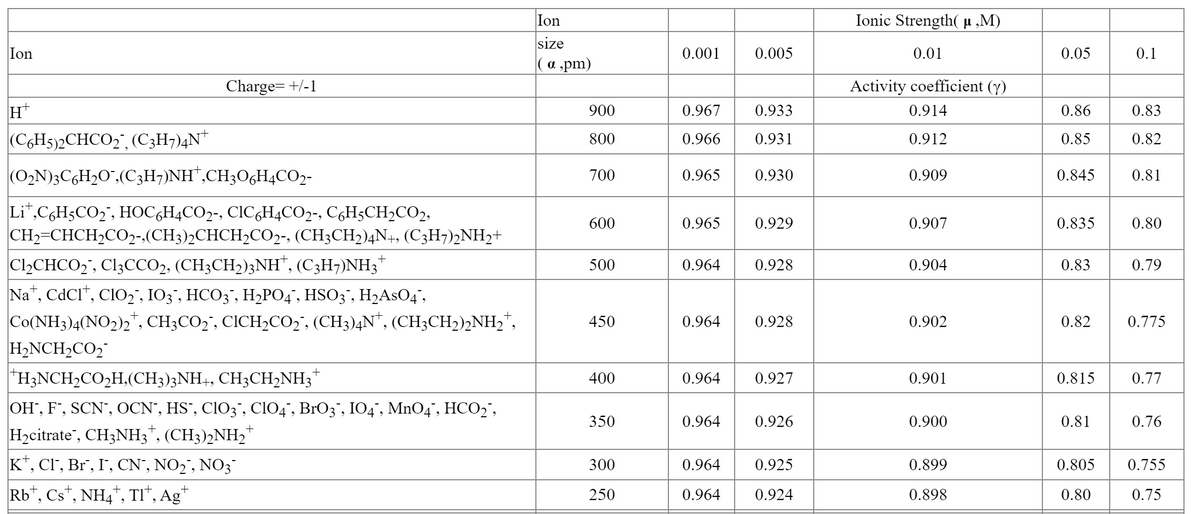
Transcribed Image Text:Ionic Strength( µ „M)
Ion
size
| ( αpm)
Ion
0.001
0.005
0.01
0.05
0.1
Charge= +/-1
Activity coefficient (y)
H
900
0.967
0.933
0.914
0.86
0.83
(C6H5)2CHCO2", (C3H7)4N*
800
0.966
0.931
0.912
0.85
0.82
(O2N)3CGH2O",(C;H¬)NH*,CH3O6H4CO2-
700
0.965
0.930
0.909
0.845
0.81
Li",C,H5CO2", HOC,H4CO2-, CIC&H4CO2-, CgH5CH2CO2,
600
0.965
0.929
0.907
0.835
0.80
CH2=CHCH2CO2-.(CH3)2CHCH2CO2-, (CH3CH2)4N+, (C3H7)2NH2+
C12CHCO2", C13CCO2, (CH3CH2);NH", (C3H7)NH3*
Na", CdCI", CIO2", I03', HCO3", H2PO4', HSO3", H2ASO4',
Co(NH3)4(NO2)2*, CH;CO2', CICH2CO2', (CH3)4N*, (CH3CH2)½NH2",
H2NCH2CO2
"H3NCH2CO2H,(CH3)3NH+, CH3CH,NH3*
500
0.964
0.928
0.904
0.83
0.79
450
0.964
0.928
0.902
0.82
0.775
400
0.964
0.927
0.901
0.815
0.77
OH", F', SCN", OCN", HS`, CIO3 , CIO4", BrO3", IO4 , MnO4", HCO2",
H2citrate", CH3NH3", (CH3)2NH2"
K*, CI, Br", I, CN", NO2", NO3
350
0.964
0.926
0.900
0.81
0.76
300
0.964
0.925
0.899
0.805
0.755
Rb*, Cs*, NH4", TI“, Ag*
0.924
0.75
250
0.964
0.898
0.80
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

