(a) What are the requirements that should be met for a precipitation reaction to serve as the basis of a gravimetric method? 1. (b) What is "digestion"? In what ways can digestion improve the quality of an analytical precipitate? (c) A typical thermogravimetric curve for magnesium oxalate (MgC204) precipitates is given below. 18 134 226 398 478 900 Temperature, °C Describe the weight loss as a function of temperature. Select a suitable ignition temperature for the gravimetric analysis of magnesium. Explain your choice. i. Predict the possible composition of the residues collected at the chosen temperature. ii. Weight, g
(a) What are the requirements that should be met for a precipitation reaction to serve as the basis of a gravimetric method? 1. (b) What is "digestion"? In what ways can digestion improve the quality of an analytical precipitate? (c) A typical thermogravimetric curve for magnesium oxalate (MgC204) precipitates is given below. 18 134 226 398 478 900 Temperature, °C Describe the weight loss as a function of temperature. Select a suitable ignition temperature for the gravimetric analysis of magnesium. Explain your choice. i. Predict the possible composition of the residues collected at the chosen temperature. ii. Weight, g
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.5QAP
Related questions
Question
SOlve the attachment
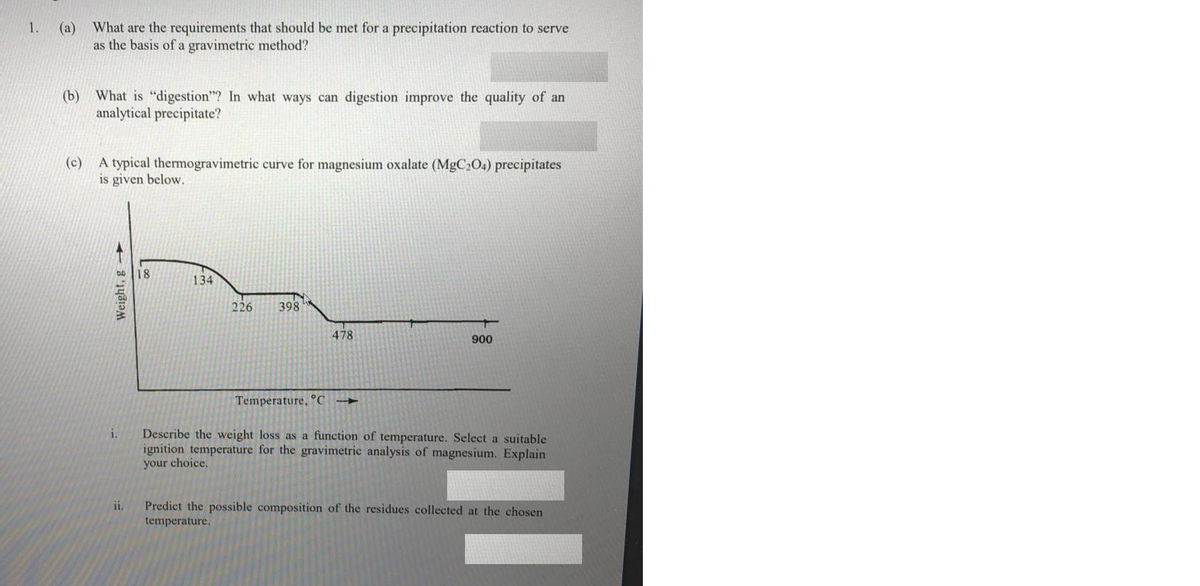
Transcribed Image Text:(a) What are the requirements that should be met for a precipitation reaction to serve
as the basis of a gravimetric method?
1.
(b) What is "digestion"? In what ways can digestion improve the quality of an
analytical precipitate?
(c) A typical thermogravimetric curve for magnesium oxalate (MgC204) precipitates
is given below.
18
134
226
398
478
900
Temperature, °C
Describe the weight loss as a function of temperature. Select a suitable
ignition temperature for the gravimetric analysis of magnesium. Explain
your choice.
i.
Predict the possible composition of the residues collected at the chosen
temperature.
ii.
Weight, g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning