Following is a retrosynthetic analysis for the synthesis of the herbicide (S)-Metolachlor from 2-ethyl-6-methylaniline, chloroacetic acid, acetone, and methanol. CI OMe OMe OMe 'N' HN N CI. + НО, Chloroacetic acid (S)-Metolachlor 3 NH2 CI + MEOH OMe Acetone Methanol 2-Ethyl-6-methylaniline Show reagents and experimental conditions for the synthesis of Metolachlor from these four organic starting materials. Your synthesis will most likely give a racemic mixture. The chiral catalyst used by Novartis for reduction in Step 2 gives 80% enantiomeric excess of the S enantiomer.
Following is a retrosynthetic analysis for the synthesis of the herbicide (S)-Metolachlor from 2-ethyl-6-methylaniline, chloroacetic acid, acetone, and methanol. CI OMe OMe OMe 'N' HN N CI. + НО, Chloroacetic acid (S)-Metolachlor 3 NH2 CI + MEOH OMe Acetone Methanol 2-Ethyl-6-methylaniline Show reagents and experimental conditions for the synthesis of Metolachlor from these four organic starting materials. Your synthesis will most likely give a racemic mixture. The chiral catalyst used by Novartis for reduction in Step 2 gives 80% enantiomeric excess of the S enantiomer.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.49P: Following is a retrosynthetic analysis for the synthesis of the herbicide (S)-Metolachlor from...
Related questions
Question
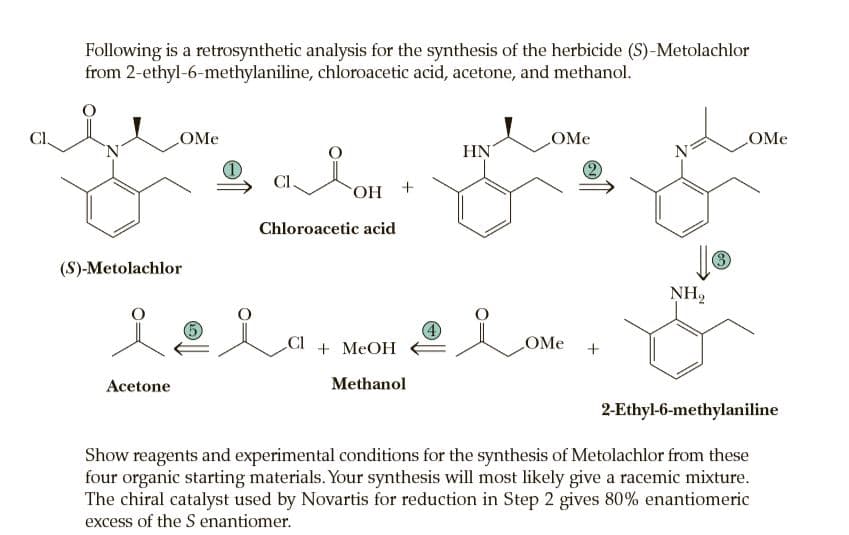
Transcribed Image Text:Following is a retrosynthetic analysis for the synthesis of the herbicide (S)-Metolachlor
from 2-ethyl-6-methylaniline, chloroacetic acid, acetone, and methanol.
CI
OMe
OMe
OMe
'N'
HN
N
CI.
+ НО,
Chloroacetic acid
(S)-Metolachlor
3
NH2
CI
+ MEOH
OMe
Acetone
Methanol
2-Ethyl-6-methylaniline
Show reagents and experimental conditions for the synthesis of Metolachlor from these
four organic starting materials. Your synthesis will most likely give a racemic mixture.
The chiral catalyst used by Novartis for reduction in Step 2 gives 80% enantiomeric
excess of the S enantiomer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning