Multistep synthesis of Lidocaine Lab 1) How many grams of starting material do you need? It is given in moles below but how many grams? 2) How to calculate theoretical yield? Part One Method A. Preparation of alpha-Chloro-2,6-dimethylacetanilideCombine 0.033 mol of 2,6-dimethylaniline with 25 mL of glacial acetic acid (or ethyl acetateor THF) and 0.033 mol of alpha-chloroacetyl chloride, in that order, in an appropriately sized Erlenmeyer flask. With the aid of a hot water bath, warm the solution to 40-50°C, remove the flask from the bath, and add a solution of 5 g of sodium acetate trihydrate dissolved in 100 mL of water.
Multistep synthesis of Lidocaine Lab 1) How many grams of starting material do you need? It is given in moles below but how many grams? 2) How to calculate theoretical yield? Part One Method A. Preparation of alpha-Chloro-2,6-dimethylacetanilideCombine 0.033 mol of 2,6-dimethylaniline with 25 mL of glacial acetic acid (or ethyl acetateor THF) and 0.033 mol of alpha-chloroacetyl chloride, in that order, in an appropriately sized Erlenmeyer flask. With the aid of a hot water bath, warm the solution to 40-50°C, remove the flask from the bath, and add a solution of 5 g of sodium acetate trihydrate dissolved in 100 mL of water.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter21: Lipids
Section: Chapter Questions
Problem 21.40P
Related questions
Question
100%
Multistep synthesis of Lidocaine Lab
1) How many grams of starting material do you need? It is given in moles below but how many grams?
2) How to calculate theoretical yield?
Part One Method
A. Preparation of alpha-Chloro-2,6-dimethylacetanilideCombine 0.033 mol of 2,6-dimethylaniline with 25 mL of glacial acetic acid (or ethyl acetateor THF) and 0.033 mol of alpha-chloroacetyl chloride, in that order, in an appropriately sized Erlenmeyer flask. With the aid of a hot water bath, warm the solution to 40-50°C, remove the flask from the bath, and add a solution of 5 g of sodium acetate trihydrate dissolved in 100 mL of water.
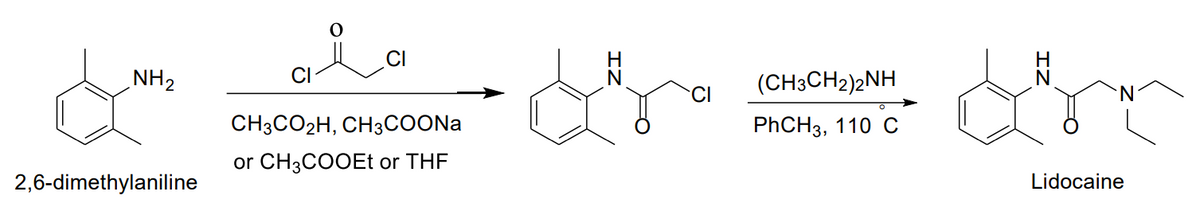
Transcribed Image Text:NH2
CI
(CH3CH2)2NH
CH3CO2H, CH3COONA
PҺCH3, 110 С
or CH3COOEt or THF
2,6-dimethylaniline
Lidocaine
IZ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning