For each ion below, draw all reasonable resonance structures (linked by resonance arrows ""). Include curved arrows that indicate the movement of electrons between each resonance structure. Assign non-zero formal charges to each atom for each resonance structure. a) NO3- (nitrate) b) CH3COO- (acetate) c) N3- (azide) d) NCO- (isocyanate)
Problem (#2.)
For each ion below, draw all reasonable resonance structures (linked by resonance arrows “↔”). Include curved arrows that indicate the movement of electrons between each resonance structure. Assign non-zero formal charges to each atom for each resonance structure.
(a.) NO3– (nitrate)
(b.) CH3COO– (acetate)
(c.) N3– (azide)
(d.) NCO– (isocyanate)
Problem (#3.)
For each ion in question 2, draw a resonance hybrid, assigning non-zero formal and/or partial charges (δ+, δ–) as needed.
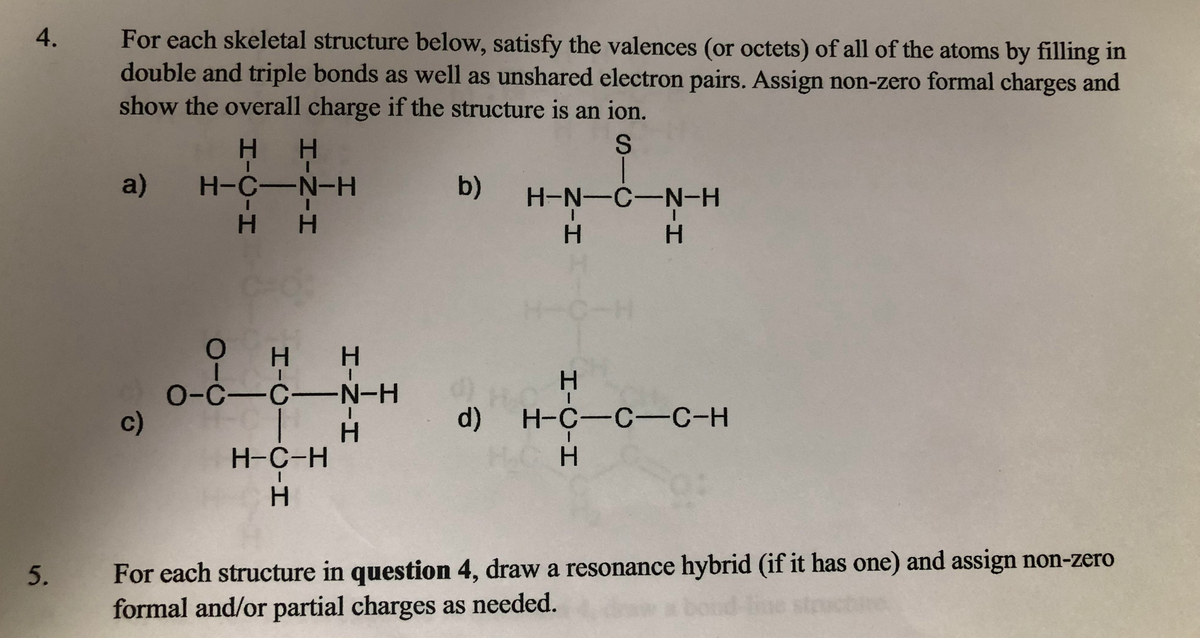
Problem (#4.)
For each skeletal structure below, satisfy the valences (or octets) of all of the atoms by filling in double and triple bonds as well as unshared electron pairs. Assign non-zero formal charges and show the overall charge if the structure is an ion.
See photo attached for Problem number 4.
Problem (#5.)
For each structure in question 4, draw a resonance hybrid (if it has one) and assign non-zero formal and/or partial charges as needed.

Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images









