Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 39P: The photoelectron spectrum of HBr has two main groups of peaks. The first has ionization energy...
Related questions
Question
For each of the following molecules draw the Lewis structure on a separate sheet of paper. MAKE SURE TO FOLLOW THE RULES FROM CLASS (ie do not break the octet rule unless necessary to connect all the atoms). These can all be drawn on one piece of paper. Based on your structure provide the following:
- the total number of valence electrons.
- a picture of the Lewis structure (3-D model not required).
- the total number of structural domains around the CENTRAL
atom. - the number of bonding domains around the CENTRAL atom.
- the number of lone pairs around the CENTRAL atom.
- the electronic and molecular shapes.
- the hybridization of the CENTRAL atom. (sp, sp2, sp3, sp3d, or sp3d2)
- whether or not the molecule is polar (Enter Y for yes and N for no).
Note: The central atom is the first atom listed.
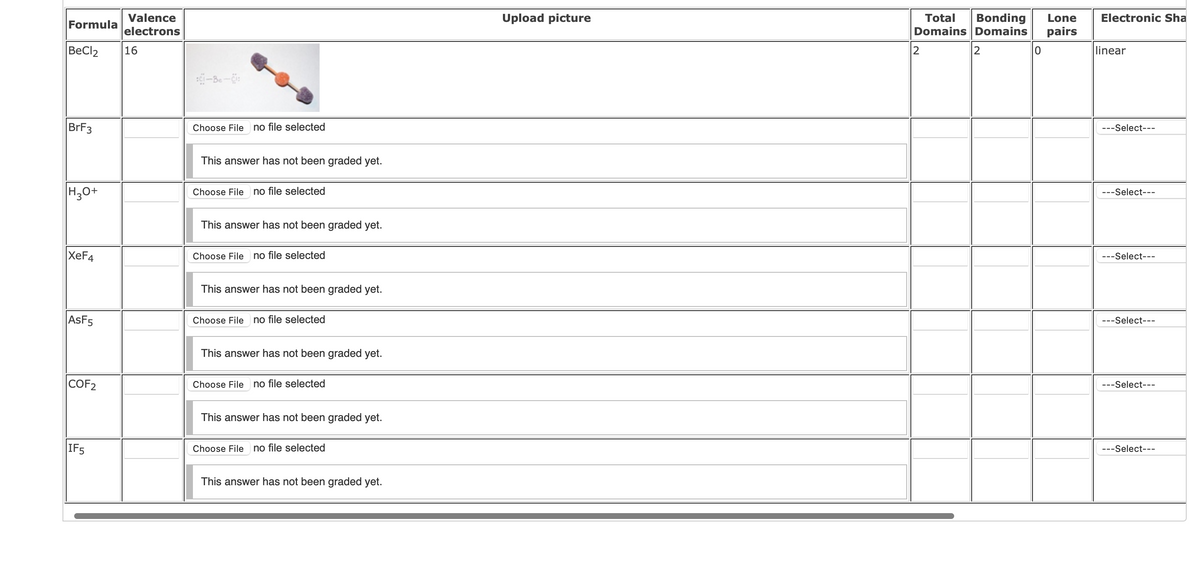
Transcribed Image Text:Valence
Upload picture
Bonding
Domains Domains
Total
Lone
Electronic Sha
Formula
electrons
pairs
BeCl2
16
2
2
linear
BrF3
no file selected
Choose File
---Select---
This answer has not been graded yet.
H30+
Choose File no file selected
---Select---
This answer has not been graded yet.
XEF4
Choose File
no file selected
---Select---
This answer has not been graded yet.
|ASF5
no file selected
Choose File
---Select---
This answer has not been graded yet.
COF2
Choose File no file selected
---Select---
This answer has not been graded yet.
IF5
Choose File
no file selected
---Select---
This answer has not been graded yet.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax