For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. iron(II) hydroxide 2. calcium sulfide Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. iron (II) hydroxide Ksp = A. NICO3 B. Ag₂CO3 C. BaF2 D. Pb(OH)2 calcium sulfide Ksp = Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.
For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. (If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. iron(II) hydroxide 2. calcium sulfide Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. iron (II) hydroxide Ksp = A. NICO3 B. Ag₂CO3 C. BaF2 D. Pb(OH)2 calcium sulfide Ksp = Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 50P
Related questions
Question
6a
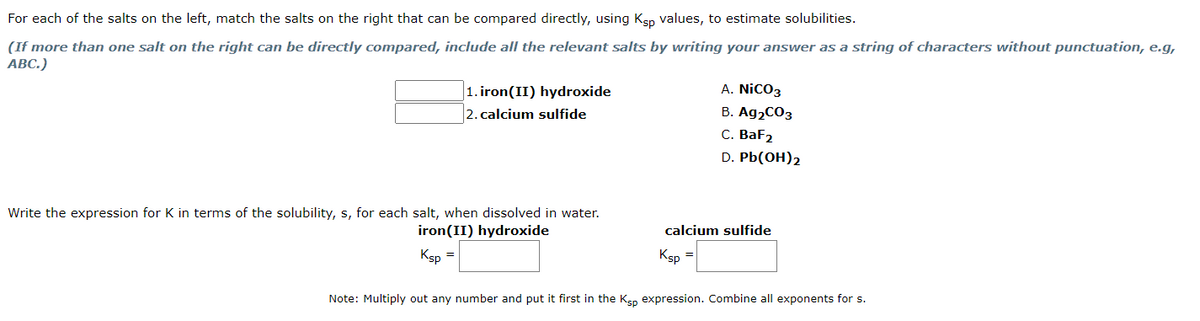
Transcribed Image Text:For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities.
(If more than one salt on the right can be directly compared, include all the relevant salts by writing your answer as a string of characters without punctuation, e.g,
ABC.)
1. iron(II) hydroxide
2. calcium sulfide
Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water.
iron (II) hydroxide
Ksp
A. NICO3
B. Ag₂CO3
C. BaF2
D. Pb(OH)2
calcium sulfide
Ksp
=
Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole