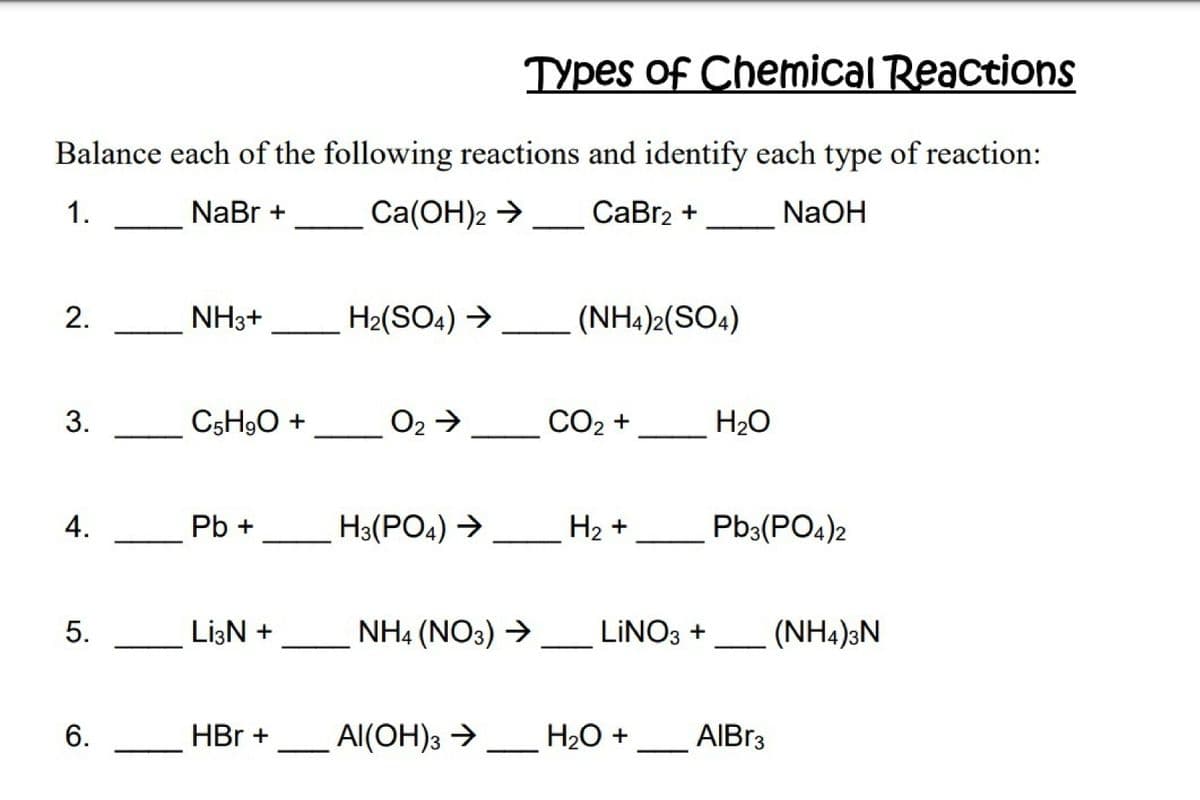
Balance each of the following reactions and identify each type of reaction: 1. NaBr + Ca(OH)2 →_ CaBr2 + NaOH 2. NH3+ H2(SO4) → (NH4)2(SO4) C5H9O + O2 > CO2 + H2O 3.
Balance each of the following reactions and identify each type of reaction: 1. NaBr + Ca(OH)2 →_ CaBr2 + NaOH 2. NH3+ H2(SO4) → (NH4)2(SO4) C5H9O + O2 > CO2 + H2O 3.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
SectionU1.7: Now You See It: The Copper Cycle
Problem 1E
Related questions
Question
Transcribed Image Text:Types of Chemical Reactions
Balance each of the following reactions and identify each type of reaction:
1.
NaBr +
Сa(ОН)2 >
СаBrz +
NaOH
2.
NH3+
H2(SO4) →
(NH4)2(SO4)
C5H9O +
O2→
СО2 +
H2O
4.
Pb +
H3(PO4) →
На +
Pb3(PO4)2
LizN +
NH4 (NO3) →
LINO3 +
(NH4)3N
6.
HBr +
Al(OH)3 →
H2O + __ AIBR3
3.
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can I see the worksheet filled in

Transcribed Image Text:Mia Jannuzzi
Types of Chemical Reactions
Balance each of the following reactions and identify each type of reaction:
1.
NaBr +
Ca(OH)2 → CaBr2 +
NaOH
2. 2 NH3+
3. 4
4.
5.
6.
H₂(SO4) →
C5H₂O
4 Cat60 + 270₂ → 20 co₂ + 18 1₂0
но
Pb3(PO4)2 S
Pb +
Li3N +
H3(PO4) →
(NH): (SO₂) Combustion
(NH4)2(SO4)
HBr + _______ Al(OH)3 →
H₂ +
NH4 (NO3)→ LINO3 +
болые rep
H₂O +
LINO3 + _______ (NH4)3N
AlBr3
double replac
Synthesis
double re
Synthesis
Identify each type of reaction:
7. Nas (PO4) + 3 KOH → 3 NaOH + K3 (PO4) double replac
8. MgCl2 + Liz(CO3) → Mg(CO₂) +2 LiCI Synthesis
9. C6H12 +9 02 →6 CO2 + 6 H₂0Combustion,
10. Pb + Fe(SO4) → Pb(SO4) + Fe double replacemer
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning