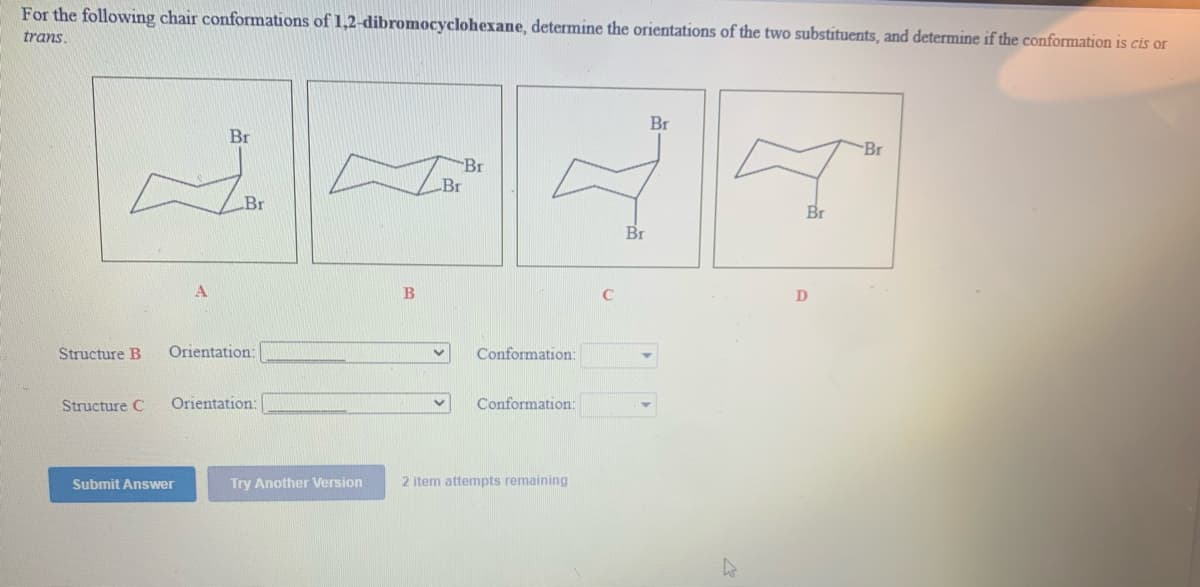
For the following chair conformations of 1,2-dibromocyclohexane, determine the orientations of the two substituents, and determine if the conformation is cis or trans. Br Br Br Br Br Br Structure B Orientation: Conformation: Structure C Orientation: Conformation:
Solution
Determining the additional stable chair conformation becomes additional advanced once there square measure 2 or additional substituents hooked up to the cyclohexane ring. to work out the stable chair conformation, the steric effects of every substituent, beside any extra steric interactions, should be taken under consideration for each chair conformations.
In this section, the result of conformations on the relative stability of disubstituted cyclohexanes is examined victimisation the 2 principles:
Substituents like equatorial instead of axial positions so as to attenuate the steric strain created of one,3-diaxial interactions.
The additional stable conformation can place the larger substituent within the equatorial position
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images






