For the following reaction, 45.5 grams of iron(II) chloride are allowed to react with 144 grams of silver nitrate iron(II) chloride(aq) + silver nitrate(aq). iron(II) nitrate(aq) + silver chloride(s) What is the maximum amount of iron(II) nitrate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams
For the following reaction, 45.5 grams of iron(II) chloride are allowed to react with 144 grams of silver nitrate iron(II) chloride(aq) + silver nitrate(aq). iron(II) nitrate(aq) + silver chloride(s) What is the maximum amount of iron(II) nitrate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section3.7: Limiting Reactants
Problem 3.18E: Urea is used as a fertilizer because it can react with water to release ammonia, which provides...
Related questions
Question
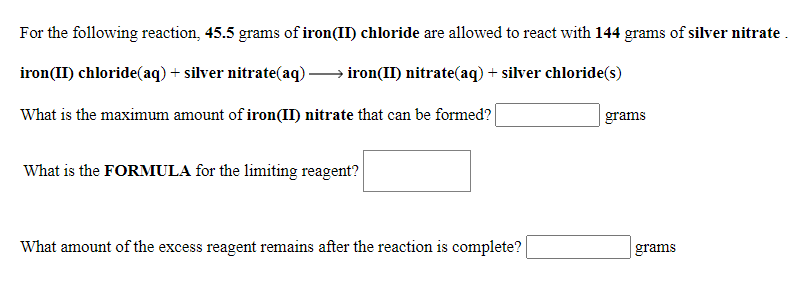
Transcribed Image Text:For the following reaction, 45.5 grams of iron(II) chloride are allowed to react with 144 grams of silver nitrate .
iron(II) chloride(aq) + silver nitrate(aq).
iron(II) nitrate(aq) + silver chloride(s)
What is the maximum amount of iron(II) nitrate that can be formed?
grams
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?
| grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning