For the gas phase isomerization of methyl cis-cinnamate, cis-C,H;CH=CHCOOCH;→trans-C&H;CH=CHCOOCH; the rate constant in s has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09×10“ K and a y-intercept of 24.3. The value of the rate constant for the gas phase isomerization of methyl cis-cinnamate at 671 K is (Enter your answer to one significant figure.) Submit Answer Retry Entire Group 9 more group attempts remaining
For the gas phase isomerization of methyl cis-cinnamate, cis-C,H;CH=CHCOOCH;→trans-C&H;CH=CHCOOCH; the rate constant in s has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09×10“ K and a y-intercept of 24.3. The value of the rate constant for the gas phase isomerization of methyl cis-cinnamate at 671 K is (Enter your answer to one significant figure.) Submit Answer Retry Entire Group 9 more group attempts remaining
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 113QRT
Related questions
Question
I don’t know how to do this question about rate constant
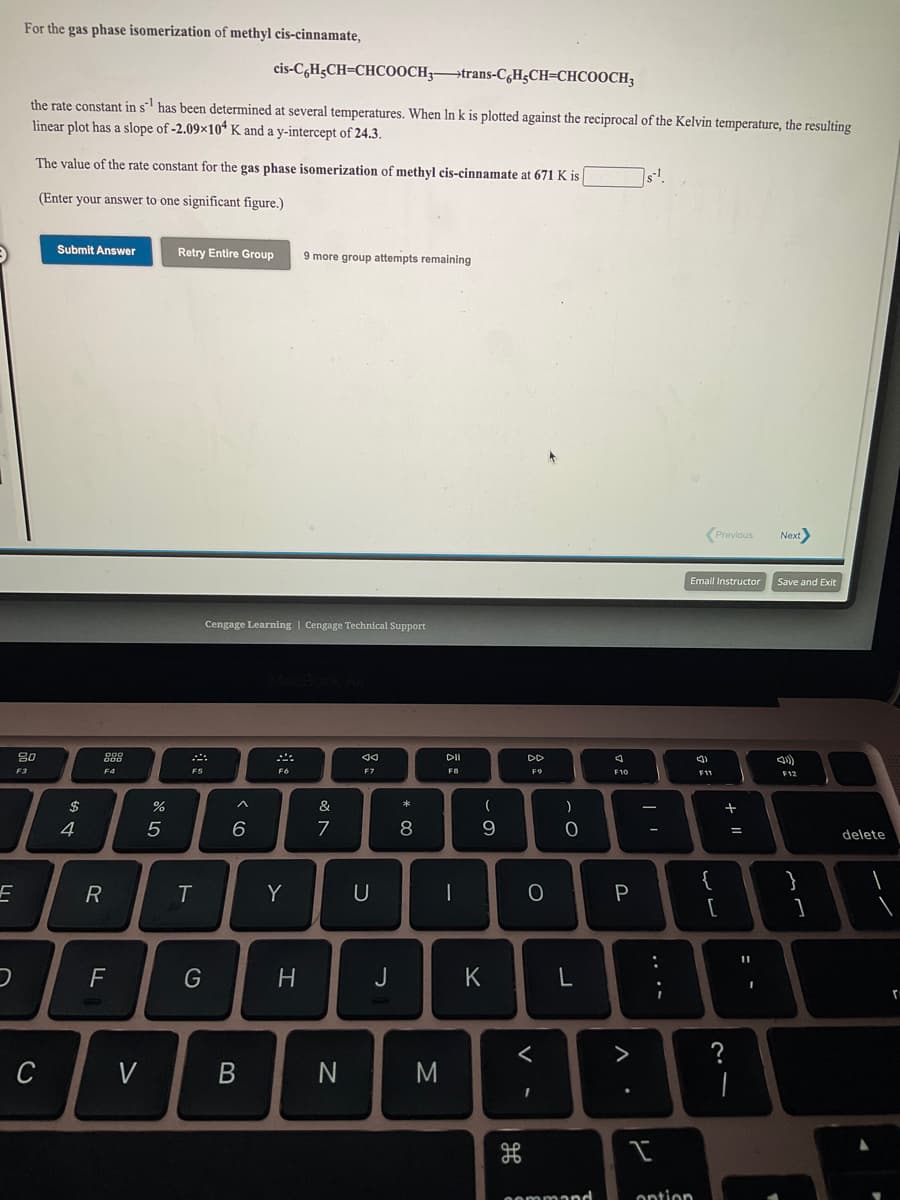
Transcribed Image Text:For the gas phase isomerization of methyl cis-cinnamate,
cis-C¿H;CH=CHCOOCH;trans-C,H;CH=CHCOOCH3
the rate constant in s has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting
linear plot has a slope of -2.09×10* K and a y-intercept of 24.3.
The value of the rate constant for the gas phase isomerization of methyl cis-cinnamate at 671 K is
(Enter your answer to one significant figure.)
Submit Answer
Retry Entire Group
9 more group attempts remaining
(Previous
Next
Email Instructor
Save and Exit
Cengage Learning | Cengage Technical Support
80
DI
DD
F3
F6
FB
F4
F5
F7
F9
F10
F11
F12
&
+
4
6
8
delete
{
}
R
Y
U
[
כ
H.
J
K
>
?
V
В
M
nommand
ontion
+ ||
ا بها
V
ト
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning