Gas cylinders, each containing 30 kg of a mixture of 75 mol% acetylene (C2H2) and balance N2 are available. How many cylinders are needed to produce 1.5 m3 of the gas mixture at 200 atm (abs) and 150°C?
Gas cylinders, each containing 30 kg of a mixture of 75 mol% acetylene (C2H2) and balance N2 are available. How many cylinders are needed to produce 1.5 m3 of the gas mixture at 200 atm (abs) and 150°C?
Given information:
Mass of gas contained in 1 cylinder, m = 30 kg
Mole% of acetylene, xA = 75%
Mole% of nitrogen, xN = 25%
Volume of gas mixture needed, V = 1.5 m3 = 1500 L
Pressure of the gas mixture required, P = 20 atm
Temperature of the gas mixture required, T = 150°C = 423 K
Let the gas mixture behave as an ideal gas. Apply an ideal gas equation to calculate the moles contained in the 1500 L gas mixture at 423 K and 200 atm. Use the value of universal gas constant (R) as 0.08206 (atm.L)/(mol.K).
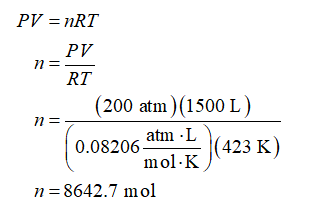
For 75 mol% acetylene and 25 mol% nitrogen, moles of acetylene and nitrogen present in 8642.7 moles of the gas mixture will be:
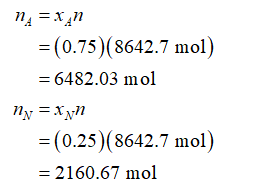
Step by step
Solved in 5 steps with 6 images









