Gibbs Free Energy using equilibrium solution composition data is calculation results in the unit joule has a natural log component in the calculation are calculated using Farraday's constant calculated using the amount of all species in the reaction. calculated using the concentration of all species in solution that have significant changes in concentration over the time studied. are calculated using redox cell potential measured in volts has temperature in Kelvin in the calculation uses the ideal gas constant in latm/molK in the calculation uses the ideal gas constant in J/Kmol in the calculation calculated using the concentration of all solid species in solution that have insignificant changes in concentration over the time studied.
Gibbs Free Energy using equilibrium solution composition data is calculation results in the unit joule has a natural log component in the calculation are calculated using Farraday's constant calculated using the amount of all species in the reaction. calculated using the concentration of all species in solution that have significant changes in concentration over the time studied. are calculated using redox cell potential measured in volts has temperature in Kelvin in the calculation uses the ideal gas constant in latm/molK in the calculation uses the ideal gas constant in J/Kmol in the calculation calculated using the concentration of all solid species in solution that have insignificant changes in concentration over the time studied.
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 10P
Related questions
Question
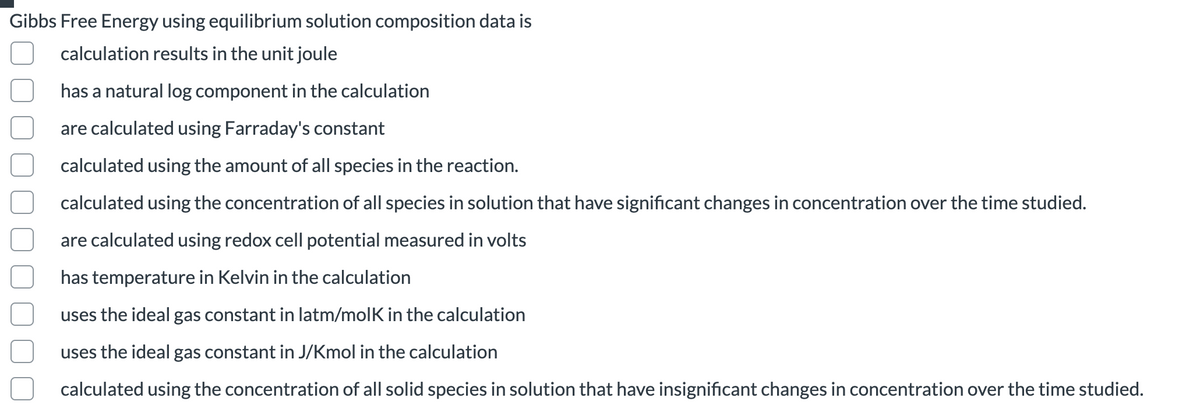
Transcribed Image Text:Gibbs Free Energy using equilibrium solution composition data is
calculation results in the unit joule
has a natural log component in the calculation
are calculated using Farraday's constant
calculated using the amount of all species in the reaction.
calculated using the concentration of all species in solution that have significant changes in concentration over the time studied.
are calculated using redox cell potential measured in volts
has temperature in Kelvin in the calculation
uses the ideal gas constant in latm/molK in the calculation
uses the ideal gas constant in J/Kmol in the calculation
calculated using the concentration of all solid species in solution that have insignificant changes in concentration over the time studied.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning