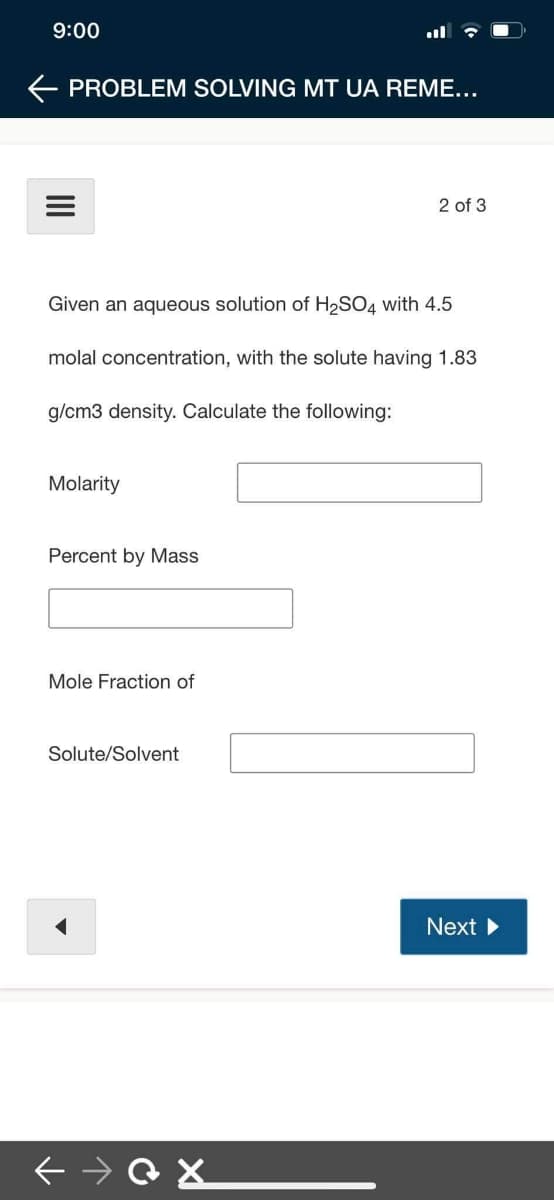
Given an aqueous solution of H2SO4 with 4.5 molal concentration, with the solute having 1.83 g/cm3 density. Calculate the following: Molarity Percent by Mass Mole Fraction of Solute/Solvent
Given an aqueous solution of H2SO4 with 4.5 molal concentration, with the solute having 1.83 g/cm3 density. Calculate the following: Molarity Percent by Mass Mole Fraction of Solute/Solvent
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
please solve asap
Transcribed Image Text:9:00
E PROBLEM SOLVING MT UA REME...
2 of 3
Given an aqueous solution of H2SO4 with 4.5
molal concentration, with the solute having 1.83
g/cm3 density. Calculate the following:
Molarity
Percent by Mass
Mole Fraction of
Solute/Solvent
Next
Transcribed Image Text:9:00
E PROBLEM SOLVING MT UA REME...
3 of 3
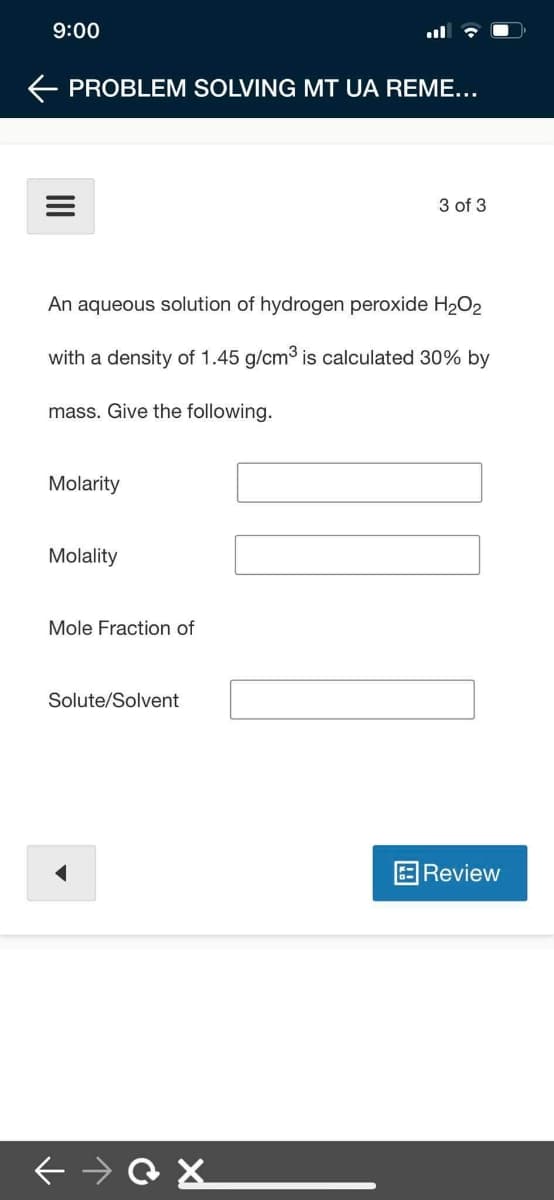
An aqueous solution of hydrogen peroxide H2O2
with a density of 1.45 g/cm3 is calculated 30% by
mass. Give the following.
Molarity
Molality
Mole Fraction of
Solute/Solvent
Review
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



