Given that at 298.15K the standard enthalpies of formation (AH,) of nitric oxide is 90.25 kJ and nitrogen dioxide is 33.18 kJ, calculate: (a) The enthalpy change for the following reaction at 298.15K 2NO(g) + 02 (g) → 2NO2 (g) at 208 1 EK
Given that at 298.15K the standard enthalpies of formation (AH,) of nitric oxide is 90.25 kJ and nitrogen dioxide is 33.18 kJ, calculate: (a) The enthalpy change for the following reaction at 298.15K 2NO(g) + 02 (g) → 2NO2 (g) at 208 1 EK
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 1.1ACP: The standard enthalpies of formation of KNO3(s) and K2S(s) are 494.6 kJ/mol and 376.6 kJ/mol,...
Related questions
Question
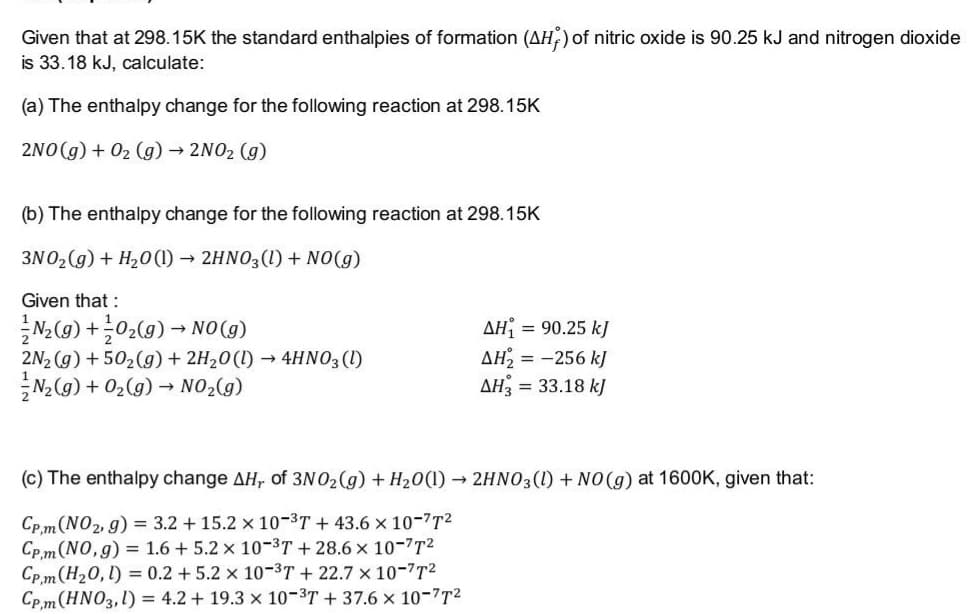
Transcribed Image Text:Given that at 298.15K the standard enthalpies of formation (AH,) of nitric oxide is 90.25 kJ and nitrogen dioxide
is 33.18 kJ, calculate:
(a) The enthalpy change for the following reaction at 298.15K
2NO(g) + 02 (g) → 2NO2 (g)
(b) The enthalpy change for the following reaction at 298.15K
3N02 (g) + H20 (1) → 2HNO3(1) + NO(g)
Given that :
(6)ON - (6)*0+ (6)'w
2N2 (g) + 502(g) + 2H20(1) → 4HNO3 (1)
N2(g) + 02(g) → NO2(g)
AH
AH2 = -256 kJ
AH3 = 33.18 kJ
= 90.25 kJ
(c) The enthalpy change AH, of 3N O2(g) + H20(1) → 2HNO3(1) + NO(g) at 1600K, given that:
Cp.m(NO2, g) = 3.2 + 15.2 x 10-3T + 43.6 x 10-7T2
Cp. m (NO,g) = 1.6 + 5.2 x 10-3T + 28.6 x 10-7T2
Cp. m (H20, l) = 0.2 +5.2 x 10-3T + 22.7 × 10-7T2
Cp,m(HNO3,1) = 4.2 + 19.3 x 10-3T + 37.6 x 10-7T2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,