Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
given the following info please provide a strucutre, the one I gave is incorrect.
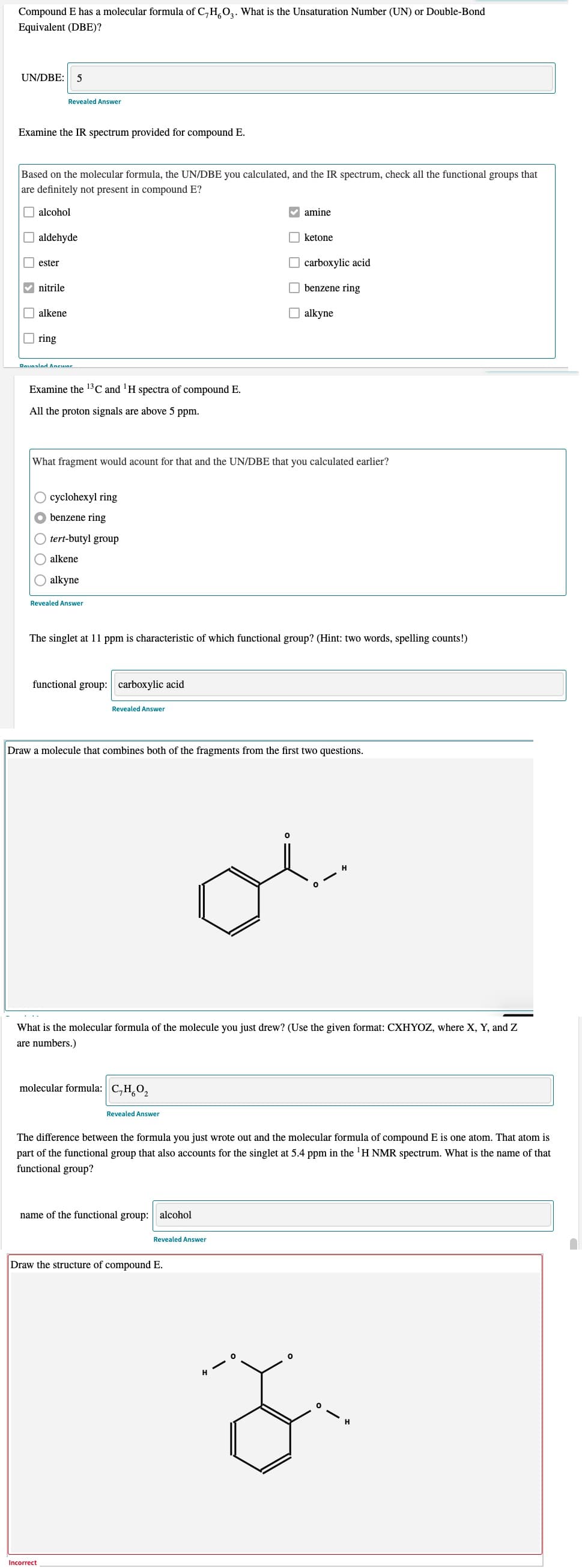
Transcribed Image Text:Compound E has a molecular formula of C,H,0,. What is the Unsaturation Number (UN) or Double-Bond
Equivalent (DBE)?
UN/DBE:
5
Revealed Answer
Examine the IR spectrum provided for compound E.
Based on the molecular formula, the UN/DBE you calculated, and the IR spectrum, check all the functional groups that
are definitely not present in compound E?
O alcohol
v amine
aldehyde
O ketone
ester
O carboxylic acid
V nitrile
O benzene ring
alkene
O alkyne
O ring
Devealed AneIaer
Examine the 13C and 'H spectra of compound E.
All the proton signals are above 5 ppm.
What fragment would acount for that and the UN/DBE that you calculated earlier?
O cyclohexyl ring
benzene ring
tert-butyl group
alkene
alkyne
Revealed Answer
The singlet at 11 ppm is characteristic of which functional group? (Hint: two words, spelling counts!)
functional group: carboxylic acid
Revealed Answer
Draw a molecule that combines both of the fragments from the first two questions.
What is the molecular formula of the molecule you just drew? (Use the given format: CXHYOZ, where X, Y, and Z
are numbers.)
molecular formula: C,H,0,
Revealed Answer
The difference between the formula you just wrote out and the molecular formula of compound E is one atom. That atom is
part of the functional group that also accounts for the singlet at 5.4 ppm in the 'H NMR spectrum. What is the name of that
functional group?
name of the functional group: alcohol
Revealed Answer
Draw the structure of compound E.
Incorrect
O D O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY