Chapter9: Energy For Today
Section: Chapter Questions
Problem 51E: The second law of thermodynamics has been called the arrow of time. Explain why this is so.
Related questions
Question
Given the following reaction mechanism:
- What is the overall reaction?
- Identify the catalyst.
- Identify the intermediates.
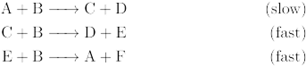
Transcribed Image Text:A + B → C+D
(slow)
C + B → D+ E
(fast)
E + B → A + F
(fast
Expert Solution
Step 1
A catalyst is a species that is present at the beginning of a reaction and reappears at the end. It does not appear in the final equation.
An intermediate is not present at the beginning. It forms during the reaction and disappears before the end. It does not appear in the final equation.
Let's apply these concepts to your mechanism.
A+B ----> C+D (slow)
C+B ----> D+E (fast)
E+B ----> A+F (fast)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning