Given the following reactions: Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- --> H2 Redox: 2NO2(g) + 2 H2O (l) --> 2NO3-(aq) + 2H+(aq) + H2(g) Conditions: [0.15 atm] [0.18 atm] [0.1 M] [0.1 M] [0.67 atm] Find the cell potential, E Report your answer in 2 decimal places. Do not include the unit
Given the following reactions: Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- --> H2 Redox: 2NO2(g) + 2 H2O (l) --> 2NO3-(aq) + 2H+(aq) + H2(g) Conditions: [0.15 atm] [0.18 atm] [0.1 M] [0.1 M] [0.67 atm] Find the cell potential, E Report your answer in 2 decimal places. Do not include the unit
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.59QE
Related questions
Question
Given the following reactions:
Anode: NO3- + 2H+ + e- --> NO2 + H2O
Cathode: 2H+ + 2e- --> H2
Redox: 2NO2(g) + 2 H2O (l) --> 2NO3-(aq) + 2H+(aq) + H2(g)
Conditions: [0.15 atm] [0.18 atm] [0.1 M] [0.1 M] [0.67 atm]
Find the cell potential, E
Report your answer in 2 decimal places. Do not include the unit
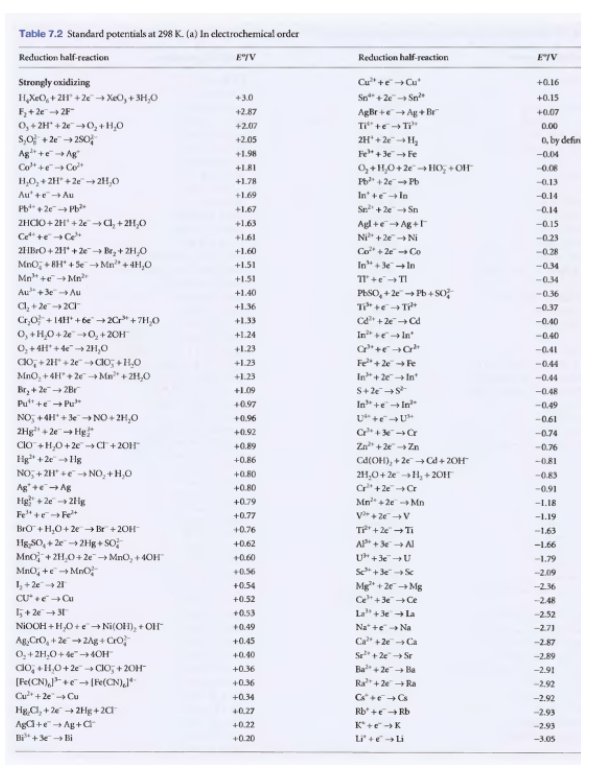
Transcribed Image Text:Table 7.2 Standard potentials at 298 K. (a) In electrochemical order
Reduction half reaction
E"/V
Reduction half-reaction
EIV
Strongly oxidizing
Cu" +eCu
+0.16
+3.0
Sn" + 2e
Sn
H,XeO, + 2H" +2e→ XeO, + 3H,O
F+2e2F"
0,+ 2H" + 2-0, + H,0
S,여 +2e→2s0어
Ag +e Ag
Co" +eCo"
+0.15
AgBr +e Ag + Br
Ti" +eTi"
2H + 2eH,
+2.87
+0.07
+2.07
0.00
+2.05
a, by defin
+1.98
Fe" + 3Fe
-0.04
0,+H,0+ 2e° -> HO; +Of"
Fb + 2e Pb
+L8I
-0.08
H,O, + 2H" +2e 21,0
Au' +eAu
Pb + 2e Pb
2HCIO + 2H' + 2e a, + 211,0
Cet +eCe
21IBrO+ 211 + 2e Br,+ 2H0
+1.78
-0.13
In +eIn
Sn+2e Sn
Agl +e Ag+
Ni" +2e Ni
Co" + 2eCo
In" + eIn
+1.69
-0.14
+1.67
-0.14
+1.63
-0.15
+1.61
-0.23
+1.60
-0.28
Mno, + BH" + Se Mn" + 4H,0
Mn" +eMn
Au" +3eAu
+1.51
-0.34
+1.51
1 +e TI
-0.34
+1.40
PESO, + 2e Pb + So
-0.36
+1.36
-0.37
Cr,0 +14H* +6e →2Cr" +7H,0
0, +H,0 + 2e" -→O, + 20H
O, +4H' + 4e 2H,0
Co, + 2H" + 2e-do; +H,0
MnO, + 4H + 2e - Ma" + 21,0
Br, + 2e 2Br
Pu" +e Pu"
+1.33
C +2eCd
-0.40
+1.24
Ine In
-0.40
+1.23
-041
Fe+ 2eFe
In + 2e In
S+2es
+1.23
-0.44
+1.23
-0.44
+1.09
-0.48
+0.97
In" +e In
-0.49
NO, +4H + 3e -NO+ 2H,0
2Hg" + 2e Hg
ClO +H,0+2e Cr + 20H
Hg" + 2e - Hg
NO, + 2H +e- NO, + H,0
+0.96
-0.61
+0.92
C+ Cr
-0.74
+0.89
Za" + 2 Zn
-0.76
+0.86
Ca(OH), + 2e-Cd + 20H
+0.80
21,0+ 2e-1, + 2011
-0.83
Ag+eAg
Hg" + 2e 21ig
Fe" +eFe*
BrO + H,0+ 2e Br + 20H
Hg,SO, + 2e -2Hg + SO?
Mno + 2H,0+ 2e"→MNO, + 40H
MnO, +e Mno
1+2e 21
+0.80
C*+2eCr
-0.91
+0.79
Mn +2e Mn
-L.18
+0.77
V+ 2eV
-1.19
+0.76
-1.63
+0.62
AP + 3e A
-1.66
+0.60
U+3e U
-1.79
+0.56
Se+3eS
-2.09
Mg* + 2e Mg
Ce" + 3eCe
+0.54
-236
+0.52
-2.48
5+ 2e31
NIOOH +H,0+e→ Ni(OH), + OH
Ag,CrO, + 2e 2Ag + CrO
O,+ 2H,0+ de-4OH
do, +1,0+2e CIO; + 20H
(Fe{CN),* +e" → [Fe(CN),J*
Cu + 2eCu
La" + 3ela
Na* +e Na
Ca +2eCa
Se + 2e Sr
+0.53
-2.52
+0.49
-271
+0.45
-2.87
+0.40
-2.89
+0.36
Ba" + 2e+ Ba
-2.91
+0.36
Ra+2eRa
-2.92
+0.34
-2.92
HgCI, + 2e + 2Hg + 20
Aga+e Ag + a
Bi +e Bi
40.27
Rb +e Rb
-2.93
+0.22
K*+eK
-2.93
+0.20
L +eLi
-3.05
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning