Given the reaction: PCI5) +PClat + Cl2g), which takes place in a 3.0L container at 573 K. 0.60 moles of PCls are initially placed in the vessel in order to react. At equilibrium, the concentration of Cl, was measured to be 0.075 M. What is the Kc of this reaction? O 0.14 O 0.045 O 0.028 O 0.011
Given the reaction: PCI5) +PClat + Cl2g), which takes place in a 3.0L container at 573 K. 0.60 moles of PCls are initially placed in the vessel in order to react. At equilibrium, the concentration of Cl, was measured to be 0.075 M. What is the Kc of this reaction? O 0.14 O 0.045 O 0.028 O 0.011
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 115IP: For the reaction NH3(g)+H2S(g)NH4HS(s) K = 400. at 35.0C. If 2.00 moles each of NH3, H2S, and NH4HS...
Related questions
Question
100%
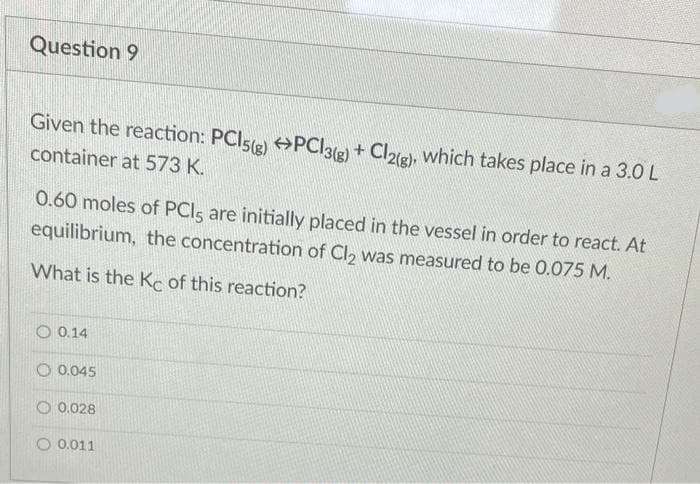
Transcribed Image Text:Question 9
Given the reaction: PCI5(e) PCI3(e) +
+ Cl2(g), which takes place in a 3.0 L
container at 573 K.
0.60 moles of PCIS are initially placed in the vessel in order to react. At
equilibrium, the concentration of Cl, was measured to be 0.075 M.
What is the Ke of this reaction?
O 0.14
O 0.045
O 0.028
O 0.011
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning