> HY > HZ Consider three generic acids with the relative stengths: HX Arrange their conjugate bases from strongest base to weakest base. Strongest base Weakest base help about us contact us terms of use privacy policy careers 99+ DELL Weakest base Answer Bank privacy polic about us careers 99+ DELL
> HY > HZ Consider three generic acids with the relative stengths: HX Arrange their conjugate bases from strongest base to weakest base. Strongest base Weakest base help about us contact us terms of use privacy policy careers 99+ DELL Weakest base Answer Bank privacy polic about us careers 99+ DELL
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.82E
Related questions
Question

Transcribed Image Text:> HY > HZ
Consider three generic acids with the relative stengths: HX
Arrange their conjugate bases from strongest base to weakest base.
Strongest base
Weakest base
help
about us
contact us
terms of use
privacy policy
careers
99+
DELL
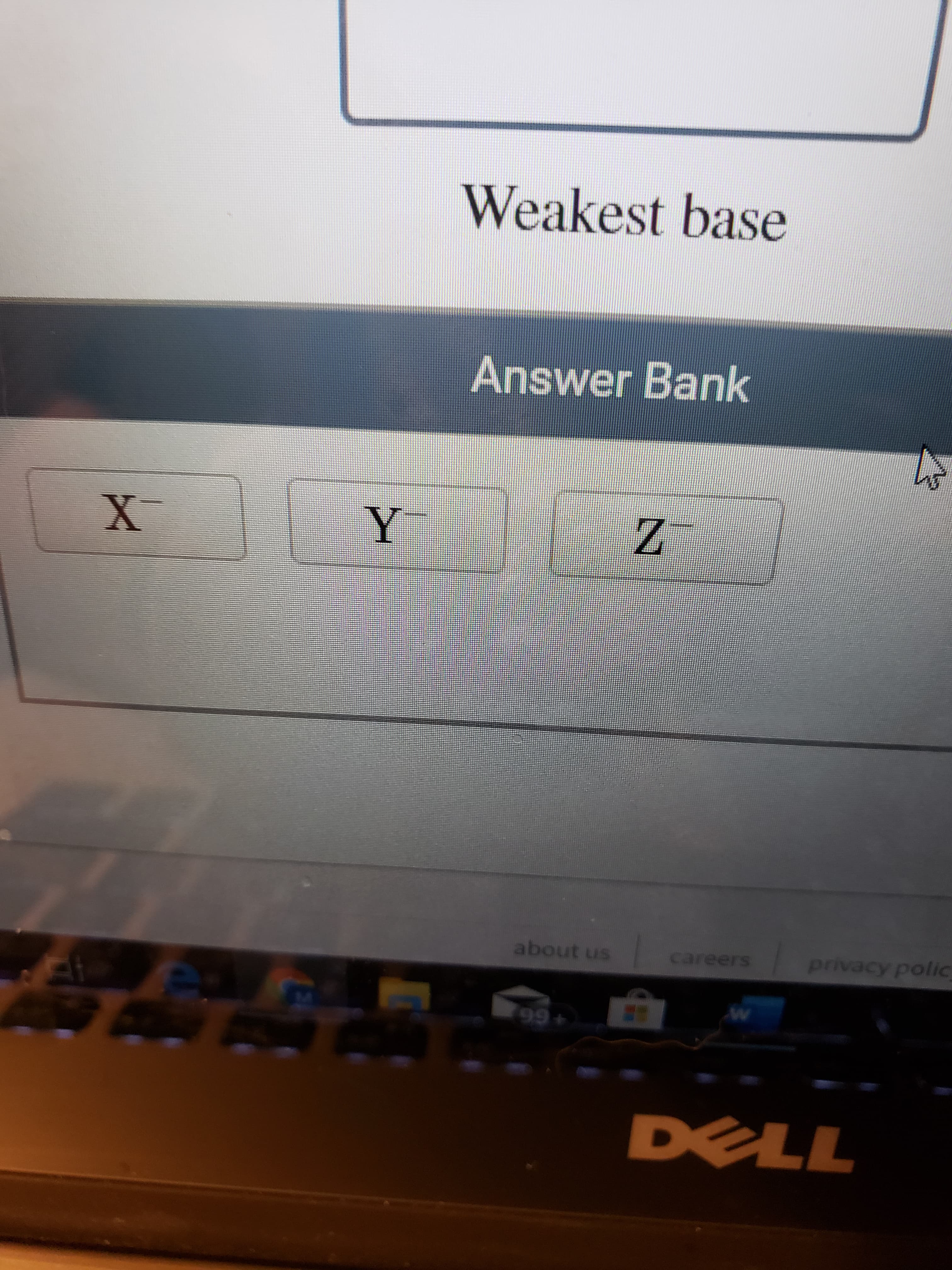
Transcribed Image Text:Weakest base
Answer Bank
privacy polic
about us
careers
99+
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning