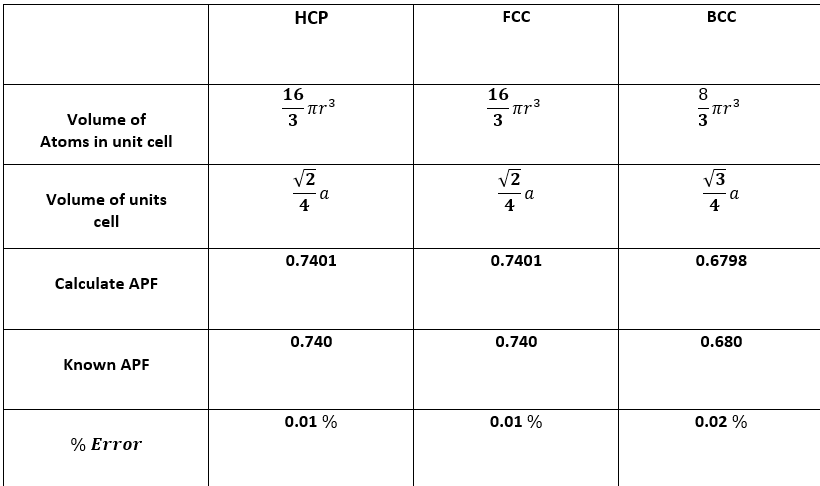
Guided Question:(Pls Answers all this Question.) 1. In crystal growth, what is the purpose of the seed crystal? 2. What is the importance of leaving the crystal growth undisturbed? How are imperfections formed in crystal growth? Cite some examples. 3. Compare and contrast the two types of close-packing in solids? 4. Why is there % error in part II? What could be the reason for such error? 5. By understanding the structure of crystals molecularly, what is its relation to the strength of materials? 6. Use the internet to search three (3) examples of metals which have HCP, FCC and BCC structures. Relate the structure to the characteristic properties of each metal. Please indicate the condition the given structures are observed.
Guided Question:(Pls Answers all this Question.)
1. In crystal growth, what is the purpose of the seed crystal?
2. What is the importance of leaving the crystal growth undisturbed? How are imperfections formed in crystal growth? Cite some examples.
3. Compare and contrast the two types of close-packing in solids?
4. Why is there % error in part II? What could be the reason for such error?
5. By understanding the structure of crystals molecularly, what is its relation to the strength of materials?
6. Use the internet to search three (3) examples of metals which have HCP, FCC and BCC structures. Relate the structure to the characteristic properties of each metal. Please indicate the condition the given structures are observed.
Step by step
Solved in 2 steps







