Halogenated compounds are particularly easy to identify by their mass spectra because chlorine and bromine occur naturally as mixtures of two abundant isotopes. • Chlorine occurs as 3ºCI (75.8%) and 37C1 (24.2%); Bromine occurs as ºBr (50.7%) and 8'Br (49.3%); • Boron compounds also stand out owing to the two isotopes 1ºB (19.9%) and "B (80.1%). For the compound Chlorocyclohexane, CgH11Cl: At what masses do the molecular ions occur? (List in order of increasing mass separated by commas, e.g. 120,122.) What are the percentages of each molecular ion? |(List to nearest 1% in order of increasing mass separated by commas, e.g. 55,45.) Visited
Halogenated compounds are particularly easy to identify by their mass spectra because chlorine and bromine occur naturally as mixtures of two abundant isotopes. • Chlorine occurs as 3ºCI (75.8%) and 37C1 (24.2%); Bromine occurs as ºBr (50.7%) and 8'Br (49.3%); • Boron compounds also stand out owing to the two isotopes 1ºB (19.9%) and "B (80.1%). For the compound Chlorocyclohexane, CgH11Cl: At what masses do the molecular ions occur? (List in order of increasing mass separated by commas, e.g. 120,122.) What are the percentages of each molecular ion? |(List to nearest 1% in order of increasing mass separated by commas, e.g. 55,45.) Visited
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.38P
Related questions
Question
how would i go about answering this
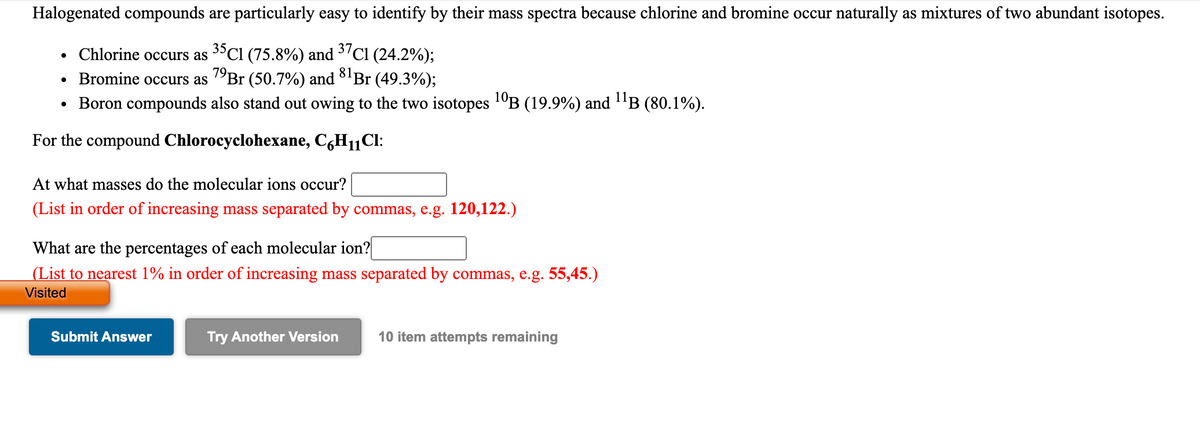
Transcribed Image Text:Halogenated compounds are particularly easy to identify by their mass spectra because chlorine and bromine occur naturally as mixtures of two abundant isotopes.
37C1 (24.2%);
• Chlorine occurs as 3°CI (75.8%) and
Bromine occurs as "Br (50.7%) and 8'Br (49.3%);
Boron compounds also stand out owing to the two isotopes 1°B (19.9%) and "B (80.1%).
81-
For the compound Chlorocyclohexane, C,H11CI:
At what masses do the molecular ions occur?
(List in order of increasing mass separated by commas, e.g. 120,122.)
What are the percentages of each molecular ion?
(List to nearest 1% in order of increasing mass separated by commas, e.g. 55,45.)
Visited
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax